- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. October 2017
ARMOR PHARMA™ lactose monohydrate 350M is a fine milled powder of α-lactose monohydrate.
Higly compressible, this grade is mostly intended to be used in tablets formulation by Wet or Dry granulation.
11. September 2017
ARMOR PHARMA - technological lactose excipients - currently markets 3 types of pharmaceutical lactose.
ARMOR PHARMA™ lactose monohydrate : sieved & milled lactose
EXCIPRESS™ lactose for Direct Compression
EXCIPURE™ lactose for Dry powder Inhalation
31. August 2017
This work aimed to obtain an optimized itraconazole (ITZ) solid oral formulation in terms of palatability and dissolution rate by combining different polymers using hot melt extrusion (HME), according to a simplex centroid mixture design. For this, the polymers Plasdone® (PVP/VA), Klucel ELF® (HPC) and Soluplus® (SOL) were processed using a laboratory HME equipment operating without recirculation at constant temperature. Samples were characterized by physicochemical assays, as well as...
31. August 2017
Systematic Design of Experiment (DoE) approach.
Simultaneous analysis of the effect of process and formulation factors.
Studying molecular level interactions via FTIR and SSNMR analyses.
30. August 2017
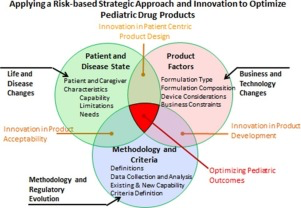
Abstract The acceptability of pediatric pharmaceutical products to patients and their caregivers can have a profound impact on the resulting therapeutic outcome. However, existing methodology and approaches used for acceptability assessments for pediatric products is fragmented, making robust and consistent product evaluations difficult. A pediatric formulation development workshop took place in Washington, DC in June 2016 through the University of Maryland’s Center of Excellence in...
30. August 2017
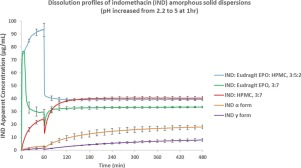
Abstract The purpose of this work was to evaluate the impact of polymer(s) on the dissolution rate, supersaturation and precipitation of indomethacin amorphous solid dispersions (ASD), and to understand the link between precipitate characteristics and redissolution kinetics. The crystalline and amorphous solubilities of indomethacin were determined in the absence and presence of hydroxypropylmethyl cellulose (HPMC) and/or Eudragit ® EPO to establish relevant phase boundaries. At acidic pH,...
30. August 2017
The rational design of drug delivery approaches leveraging supramolecular chemistry (i.e., “chemistry beyond the molecule”) has garnered significant interest in recent years toward improving therapeutics. By using specific, dynamic, and tunable non-covalent interactions, engineered approaches to drug delivery can be realized.
30. August 2017
Chemotherapy is the most common therapeutic strategy for the treatment of unresectable hepatocellular carcinoma. However, the therapeutic efficacy is limited by low delivery efficiency of chemotherapeutics and severe toxicity towards healthy tissues.
29. August 2017
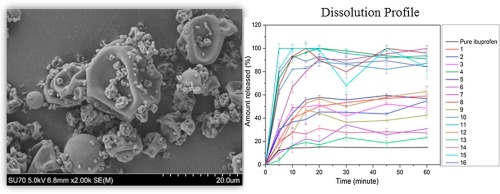
The main objective of present research investigation is to formulate the Moxi oxacin.HCl Fast Dissolving tablets. Moxi oxacin.HCl, a synthetic u- oroquinolone antibacterial agent, and used to treatacute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis
29. August 2017
Electrospinning of Eudragit® FS 100 was investigated for the first time.
• Novel AC electrospinning produced excellent quality nano fibers at high throughput.
• Melt rheology of Eudragit® FS 100 was thoroughly explored.
• Eudragit® FS-based solid dispersions provided excellent control on release of poorly soluble spironolactone as pH varied.