- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. August 2018
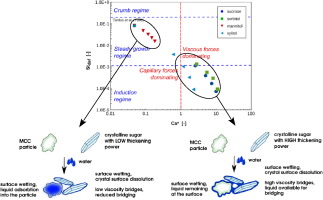
Sugars and sweeteners are common ingredients in foods and also in pharmaceutical industries. They are soluble and sticky ingredients and their processing in granulators may be difficult since they can easily adhere to mixer walls or leadto uncontrolled granule growth. The purpose of this research was to evaluate the feasibility of the high shear wet granulation process and to find the optimal amount of binder by studying the granulation performances of four sugars: mannitol, sorbitol, xylitol...
22. August 2018
The effects of excipients on the accuracy of tablet subdivision are severely underinvestigated. In this study, placebo tablets were prepared using a combined mixture design of fillers and binders to evaluate the effect of these excipients on subdivision accuracy. The responses assessed were mass loss, mass variation, tablet fragmentation, and increased friability. Dicalcium phosphate dihydrate (DCP) gave rise to more uniform and denser tablets than microcrystalline cellulose (MCC), thus...
30. April 2018
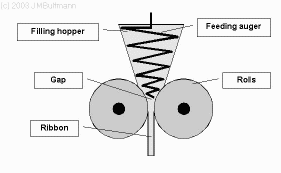
Metformin has a poor tabletability and flowability. Therefore, metformin is typically wet granulated with a binder before tableting. To save production costs, it would be desirable to implement a roll compaction/dry granulation (RCDG) process for metformin instead of using wet granulation. In order to implement RCDG, the efficiency of dry binders is crucial to ensure a high drug load and suitable properties of dry granules and tablets. This study evaluates dry granules manufactured by RCDG and...
28. March 2018
In twin screw wet granulation process, the binding excipients could be added in two ways: premixed with powder materials before granulation or dissolved in water as a solution. In this paper, the feasibility of using micronized lactose as a solid binder excipient in twin screw granulation process was examined. Different proportions of micronized lactose were mixed with lactose and microcrystalline cellulose (MCC) powder before granulation, respectively. As a comparison, hydroxypropyl cellulose...
13. March 2018
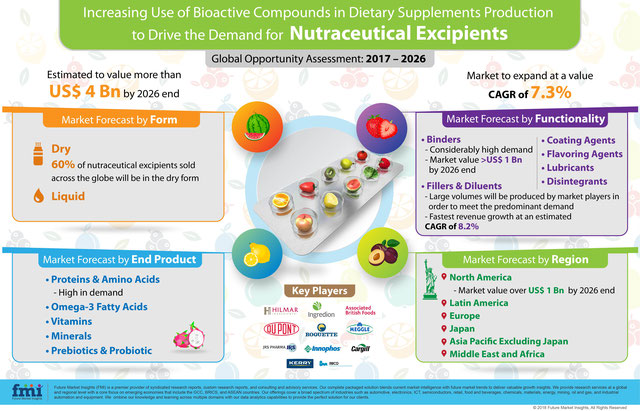
For their nutritional and medicinal advantages, several bioactive compounds and ingredients are being used in the production of medical foods, functional foods and dietary supplements. As health consciousness among consumers increases, the demand for dietary supplements will witness an upsurge. In order to attain a stabilized manufacturing of these products, a range of nutraceutical excipients will be utilized.
31. January 2018
Multiple brands of the same active ingredient may be available for the same strength, administration route and dose form. Generic brands needs to demonstrate bioequivalence to the originator brand, but the appearance of the generic and originator brands are not required to match.
04. January 2018
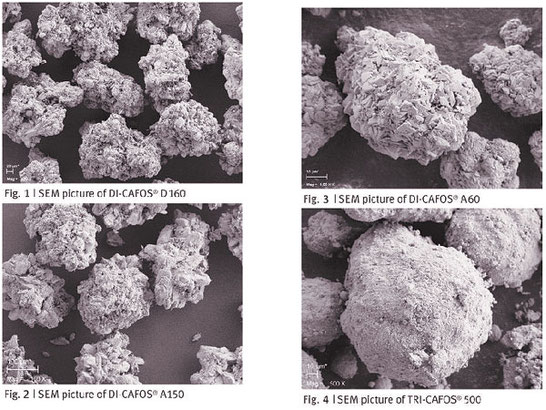
Calcium phosphates have been used in the pharmaceutical technology for many years. They have many physical and chemical properties that make them ideal candidates for the production of solid oral dosage forms. They are mainly used as fillers in order to bulk up formulations however, the function of calcium phosphates goes far beyond mere filler and the adequate utilisation of their full functionality can support achieving the intended formulation goals.
03. November 2017
In the new era of medicine, 3D printing technique in pharmaceutical manufacturing has already yielded success. For example, Aprecia®, an FDA-approved pharmaceutical company, has launched its first approved product which is not only unique because of a novel manufacturing process but also better than conventional compressed tablets.
21. October 2017
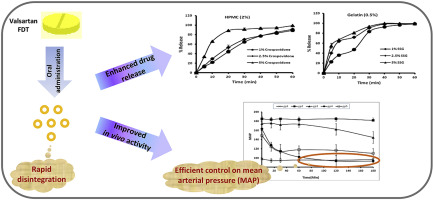
Fast disintegrating tablets (FDTs) dissolve or disintegrate in the mouth without the need of additional water. The aim of the present study was to formulate FDTs of Valsartan for the treatment of hypertension in children who could find difficulties in swallowing conventional solid dosage forms. The tablets were prepared by wet granulation technique. Superdisintegrants such as sodium starch glycolate (SSG) and crospovidone were optimized as 5% on the basis of least disintegration time. Different...
02. May 2017
Highlights
• Binder properties were correlated to granule and tablet properties.
• Groups of binders behave divergent regarding granule and tablet properties.
• Povidone based polymers seem to have another binding behaviour.
• Disintegration of tablets was explained by particle size and viscosity effects.
• Changes in particle size caused larger effects for small-sized binders on tablets.