- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. May 2018
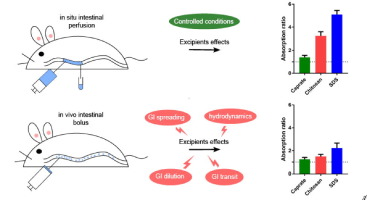
Pharmaceutical excipients that may affect gastrointestinal (GI) drug absorption are called critical pharmaceutical excipients (CPEs), or absorption-modifying excipients (AMEs) if they act by altering the integrity of the intestinal epithelial cell membrane. Some of these excipients increase intestinal permeability, and subsequently the absorption and bioavailability of the drug. This could have implications for both the assessment of bioequivalence and the efficacy of the absorption-enhancing...
17. October 2017
Dry powder inhaler for pulmonary drug delivery: human respiratory system, approved products and therapeutic equivalence guideline
04. September 2017
This work presents a review of literature and experimental data relevant to the possibility of waiving pharmacokinetic bioequivalence studies in human volunteers for approval of immediate release solid oral pharmaceutical forms containing folic acid as the single active pharmaceutical ingredient. For dosage forms containing 5 mg folic acid, the highest dose strength on the WHO Essential Medicines List, the dose/solubility ratio calculated from solubility studies was higher than 250 mL,...
25. May 2017
Abstract: Bacterial resistance and antibiotic drug effectiveness can be related to administering generic products with a subtherapeutic dose or poor in vivo drug release. The aim of this study was to investigate whether locally marketed amoxicillin tablets have the required chemical and physical attributes, including in vitro bioequivalence performance. Five generic products (T1, T2, T3, T4, and T5) containing combination of amoxicillin trihydrate and potassium clavulanate as 1 g strength...
02. May 2017
The first-in-class selective inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase type 5 (PDE5), sildenafil, has become well established as a safe and effective treatment for male erectile dysfunction since the approval of Viagra® (Pfizer Ltd, Tadworth, UK) by the US Food and Drug Administration (FDA) in 1998. Guidelines on male sexual dysfunction established by the European Association of Urology recommend oral pharmacotherapy with PDE5 inhibitors as first-line therapy.
04. April 2017
Abstract The objective of this study was to develop Esomeprazole solid dosage form as minitablets. The effect of coating thickness and percentage of fast disintegrants on the in vitro and in vivo performance of minitablets were studied. Two formulae (A1&B1) of the same core composition with different coat thickness were prepared initially. The in vivo study of A1 and B1 versus the originator revealed that their rate of dissolution was not enough to achieve bioequivalence with respect to...
24. October 2016
Abstract The rapidly growing medical expense going with aging population in Japan requires the use of generic products derived from off-patent active pharmaceutical ingredients (APIs) formulations to reduce the financial burden for the national health insurance system, while at the same time avoiding undermining the quality of medical care. This article provides an overview of the regulatory approaches available to confirm the quality of generic products and gain their greater acceptance by...
25. July 2016
Purpose In ocular drug development, an early estimate of drug behavior before any in vivo experiments is important. The pharmacokinetics (PK) and bioavailability depend not only on active compound and excipients but also on physicochemical properties of the ocular drug formulation. We propose to utilize PK modelling to predict how drug and formulational properties affect drug bioavailability and pharmacokinetics. Methods A physiologically relevant PK model based on the rabbit eye was built to...
19. July 2016
Abstract The objective of this study was to develop a novel prasugrel base microsphere-loaded tablet (PBMST) with enhanced stability as a bioequivalent to the commercial prasugrel hydrochloride-loaded tablet. Numerous prasugrel base-loaded microspheres were prepared with hydroxy-propylmethyl cellulose (HPMC), colloidal silica and various acidifying agents using a spray-drying process, and the physicochemical properties, solubility and stability were investigated. The PBMSTs were prepared and...
31. March 2016
Abstract We previously concluded that 12 common excipients need not be qualitatively the same and quantitatively very similar to reference for Biopharmaceutics Classification System–based biowaivers. This conclusion for regulatory relief is based upon a series of bioequivalence studies in humans involving cimetidine and acyclovir. Limitations were also discussed. We understand the major concern of García-Arieta et al. is that “results obtained by Vaithianathan et al. should not be...