- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
29. August 2018
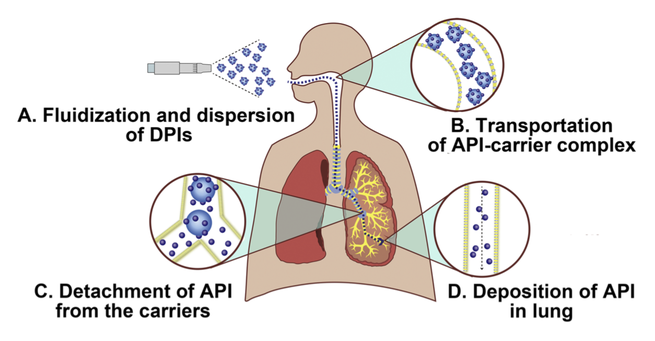
Carrier based dry powder inhalers (DPIs) are common vehicles for pulmonary delivery of active pharmaceutical ingredients (APIs), thus powder properties of carrier would generate a pronounced impact on the two dominant delivery stages of the DPIs. In our previous study, a novel modified carrier, the nanoporous mannitol carriers (NPMCs) was prepared to achieve satisfactory aerosolization performance. While the aerosolization performance enhancement mechanism of NPMCs based DPI was still...
28. August 2018
Carrier based dry powder inhalers (DPIs) are common vehicles for pulmonary delivery of active pharmaceutical ingredients (APIs), thus powder properties of carrier would generate a pronounced impact on the two dominant delivery stages of the DPIs. In our previous study, a novel modified carrier, the nanoporous mannitol carriers (NPMCs) was prepared to achieve satisfactory aerosolization performance. While the aerosolization performance enhancement mechanism of NPMCs based DPI was still...
26. May 2018
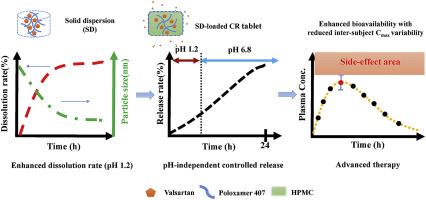
The aims of this work were to design pH-independent controlled release (CR) tablet containing nanonizing solid dispersion (SD) adsorbed on hydrophilic silica (Aeroperl® 300/30). Valsartan (VAL) was chosen to simultaneously modulate solubility and release rate due to its poor water solubility in low pH condition and short elimination half-life. Based on extensive equilibrium solubility and compatibility studies, poloxamer 407 was selected as a SD carrier. The melted mixtures of drug and...
15. April 2018
This study was performed to investigate how increasing the active pharmaceutical ingredient (API) content within a formulation affects the dispersion of particles and the aerosol performance efficiency of a carrier based dry powder inhalable (DPI) formulation, using a custom dry powder inhaler (DPI) development rig. Methods Five formulations with varying concentrations of API beclomethasone dipropionate (BDP) between 1% and 30% (w/w) were formulated as a multi-component carrier system...
15. March 2017
Abstract The formulation of lipophilic and hydrophobic compounds is a challenge for the pharmaceutical industry and it requires the development of complex formulations. Our first aim was to investigate hot-melt extrudate microstructures by means of multifractal analysis using scanning electron microscopy imaging. Since the microstructure can affect solid dosage form performance such as mechanical properties, a second objective was to study the influence of the type of adsorbent and of the...
03. February 2017
Abstract The aim of this study was to develop nanostructured lipid carriers (NLCs) for transdermal delivery of acid-labile lansoprazole (LPZ). The drug loading, particle size, zeta potential, thermal behavior and stability of NLCs were evaluated. The particle size of NLCs was in the range of 90−210 nm and the zeta potential was −61.9 to +3.2 mV dependent of the compositions. Stearylamine (SA) prevented lansoprazole degradation and maintained drug stable in NLCs. The anionic sodium dodecyl...
20. September 2016
Abstract Formation of core-shell nanocomposites of Fenofibrate and Itraconazole, model poorly water soluble drugs, via fluidized bed (FB) coating of their well-stabilized high drug loaded nanosuspensions is investigated. Specifically, the extent of dissolution enhancement, when fine carrier particles (sub–50 μm) as opposed to the traditional large carrier particles (>300 μm) are used, is examined. This allows testing the hypothesis that greatly increased carrier surface area and more...
17. July 2016
Abstract Solubility represents an important challenge for formulation of drugs, because the therapeutic efficacy of a drug depends on the bioavailability and ultimately on its solubility. Low aqueous solubility is one of the main issues related with formulation design and development of new molecules. Many drug molecules present bioavailability problems due to their poor solubility. For this reason there is a great interest in the development of new carrier systems able to enhance the...
02. May 2016
To improve the dissolution and oral bioavailability (BA) of atorvastatin calcium (ATV), we previously introduced an optimized self-microemulsifying drug delivery system (SMEDDS) using Capmul® MCM (oil), Tween® 20 (surfactant), and tetraglycol (cosurfactant). In this study, various solid carriers were employed to develop a solidified SMEDDS (S-SMEDDS): mannitol (M) and lactose (L) as water-soluble carriers, and Sylysia® 350 (S) and Aerosil® 200 (A) as water-insoluble carriers. Maximum...
26. April 2016
This study investigated the impact of macro-scale carrier surface roughness on the performance of dry powder inhaler (DPI) formulations. Methods Fluid-bed processing and roller compaction were explored as processing methods to increase the surface roughness (Ra) of lactose carrier particles. DPI formulations containing either (a) different concentrations of fine lactose at a fixed concentration of micronized drug (isoniazid) or (b) various concentrations of drug in the absence of fine lactose...