- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
06. April 2017
Abstract A stabilized high drug load intravenous formulation could allow compounds with less optimal pharmacokinetic profiles to be developed. Polyethylene glycol (PEG)-ylation is a frequently used strategy for particle delivery systems to avoid the liver, thereby extending blood circulation time. The present work reports the mouse in vivo distribution after i.v. administration of a series of nanocrystals prepared with the bead milling technique and PEG-ylated with DSPE-PEG2000 and Pluronic...
13. February 2017
Abstract In order to save time and resources in early drug development, in vitro methods that correctly predict the formulation effect on oral drug absorption are necessary. The aim of this study was to 1) evaluate various BCS class II drug formulations with in vitro methods and in vivo in order to 2) determine which in vitro method best correlates with the in vivo results. Clarithromycin served as model compound in formulations with different particle sizes and content of excipients. The...
15. December 2016
Abstract There is ample evidence that pharmaceutical excipients, which are supposed to be pharmacologically inactive, have an impact on drug metabolism and efflux transport. So far, little is known whether they also modulate uptake transporter proteins. We have recently shown that commonly used solubilizing agents exert significant effects on the function of organic anion uptake transporting polypeptides. Therefore, we investigated in this study the influence of frequently used pharmaceutical...
16. November 2016
Abstract In ocular surface inflammatory diseases, such as dry eye disease, long-term symptom relief requires targeting the inflammation itself rather than treating only the surface-associated dryness with artificial tears. Therefore, we included an anti-inflammatory agent in an unpreserved liposome-based (LP) formulation used as artificial tears. Our aim was to characterize and study its in vitro and ex vivo cell uptake and functionality. Human corneal epithelial (HCE) cells were used to study...
18. September 2016
Abstract: Background: Amphotericin B eye drops are widely used in the treatment of ocular infections. However, amphotericin’s toxicity leads to low patient compliance and aggravation of symptoms. This work describes the development of a microemulsion system containing amphotericin B, aiming for its use in ocular applications. Methods: The microemulsion was developed by the titration technique. The physicochemical characteristics were determined with both loaded and unloaded amphotericin...
07. August 2016
Abstract Hyaluronan (HA) is frequently incorporated in eye drops to extend the pre-corneal residence time, due to its viscosifying and mucoadhesive properties. Hydrodynamic and rheological evaluations of commercial products are first accomplished revealing molecular weights varying from about 360 to about 1200 kDa and viscosity values in the range 3.7–24.2 mPa s. The latter suggest that most products could be optimized towards resistance to drainage from the ocular surface. Then, a study...
02. May 2016
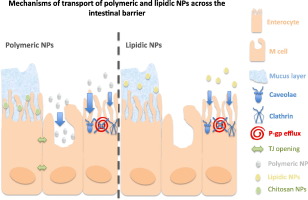
Unraveling the mechanisms of nanoparticle transport across the intestinal barrier is essential for designing more efficient nanoparticles for oral administration. The physicochemical parameters of the nanoparticles (e.g., size, surface charge, chemical composition) dictate nanoparticle fate across the intestinal barrier. This review aims to address the most important findings regarding polymeric and lipidic nanoparticle transport across the intestinal barrier, including the evaluation of...
17. December 2015
The chemotherapeutic drug substance doxorubicin has been reported to be a substrate of P-gp, which induces a barrier for oral administration and leads to a bioavailability of 3% in male Sprague Dawley rats. Literature studies have reported increased transport of P-pg substrates, like digoxin, when co-administered with P-gp inhibitors (non-ionic surfactants) in vitro and in vivo. More
13. September 2015
The purpose of the present study was to develop the Solutol HS15-based doxorubicin submicron emulsion with good stability and overcoming multi-drug resistance. In this study, we prepared doxorubicin submicron emulsion, and examined the stability after autoclaving, the in vitro cytotoxic activity, the intracellular accumulation and apoptpsis of doxorubicin submicron emulsion in MCF-7/ADR cells. The physicochemical properties of doxorubicin submicron emulsion were not significantly affected after...