- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. June 2018
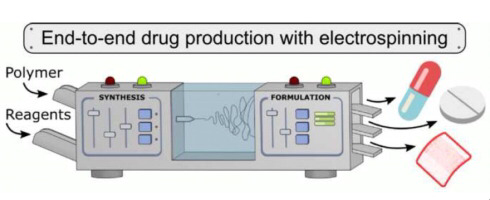
Based on the concept of continuous manufacturing an end-to-end benchtop device was developed unprecedented for the production of solid drug dosage forms by connecting flow synthesis and formulation via electrospinning (ES). Together with the optimized two-step continuous-flow synthesis of acetylsalicylic acid (ASA) a water-soluble polymeric excipient (polyvinylpyrrolidone K30, PVPK30) was introduced. The resulting polymeric solution could be readily electrospun into solid nanofibers with high...
25. April 2018
To support the practical implementation of the ICH Q3D guideline, which describes a risk-based approach to the control of elemental impurities in drug products, a consortium of pharmaceutical companies has established a database to collate the results of analytical studies of the levels of elemental impurities within pharmaceutical excipients. This database currently includes the results of 26723 elemental determinations for 201 excipients and represents the largest known, and still rapidly...
29. March 2017
ABSTRACT: Control of elemental impurities in pharmaceutical materials is currently undergoing a transition from control based on concentrations in components of drug products to control based on permitted daily exposures in drug products.Within the pharmaceutical community, there is uncertainty regarding the impact of these changes on manufactures of drug products. This uncertainty is fueled in part by a lack of publically available information on elemental impurity levels in common...
03. February 2016
The chairs of each of the 8 Special Interest Groups of the Board of Pharmaceutical Sciences of the International Pharmaceutical Federation have compiled opinions with regard to major challenges for the pharmaceutical sciences over the next 5-10 years. Areas covered are drug design and discovery, natural products, formulation design and pharmaceutical technology, pharmacokinetics /pharmacodynamics and systems pharmacology, translational and personalized medicine, biotechnology, analytical...
29. January 2016
The objective of the current research article is to provide a comprehensive review of excipients impact on the stability of the drug product and their implications during the product development. Recent developments in the understanding of the degradation pathways further impact methodologies used in the pharmaceutical industry for potential stability assessment. The formation of drug excipient adducts was very common based on the sensitive chemical moieties in the drugs and the excipients. The...