- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
24. August 2018
A process control system based on PAT can compensate for variations in particle size, resulting in more consistent coating thickness.
Drug-layered multiparticulates are a common dosage form for extended or modified-release pharmaceutical formulations. Delivered either in capsules, tablets, or as food additives in pediatric or geriatric applications (1), these formulations typically feature a functional coating designed to delay dissolution of the drug in the body.
03. April 2018
Overview Webinar Title: Streamlined Manufacture of Modified Release Matrix Tablets via Direct Compression Date: Tuesday, April 10, 2018 Time: 11:00 AM Eastern Daylight Time Duration: 1 hour
21. February 2018
The Marketing Communications Specialist reports to the Global Director of Marketing Communications and is responsible for all assigned marketing communications projects, internal and external, inclusive of literature, advertising, website content development and management, social media and automated marketing campaigns. The incumbent will provide input to market communication plans and promotional objectives while being the lead for implementation of digital marketing initiatives.
07. February 2018
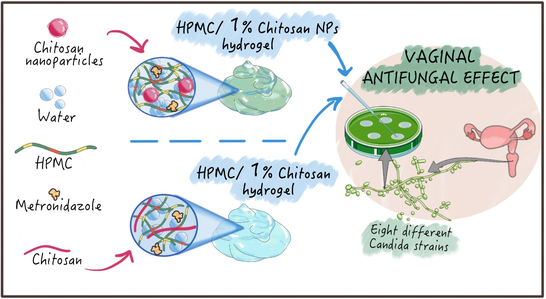
Polymeric hydrogels are common dosage forms designed for the topical administration of antimicrobial drugs to treat vaginal infections. One of the major advantages of using chitosan in these formulations is related to the intrinsic and broad antimicrobial activity exerted on bacteria and fungi by this natural polymer. Most vaginal yeast infections are caused by the pathogenic fungus Candida albicans.
24. January 2018
Considering everything involved with advancing a drug from discovery to a marketed product, the preparation of the film coating may seem like a simple step; just mixing powder and water. Yet it is an important step, and one of the essential steps we are regularly asked about.
17. January 2018
Colorcon Inc. announces an exciting process development for sugar coated pharmaceutical tablets with the launch of Opadry® SGR, Rapid Sugar Film Coating System. This new aqueous system delivers a more streamlined application process, reducing coating time from days to hours and improving manufacturing safety through the elimination of organic solvents.
12. December 2017
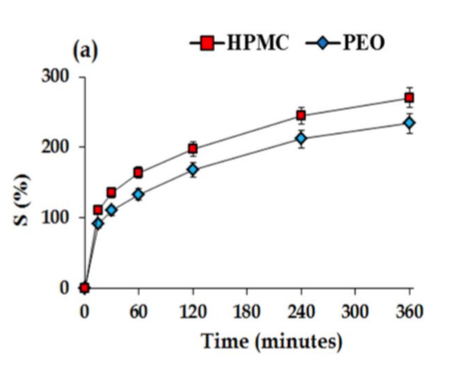
The aim of the present investigation was to understand the swelling behaviour of HPMC and PEO-based matrices and to evaluate the impact of porosity on the swelling kinetics. It was noticed that the HPMC has higher swelling rates but both undergo diffusion oriented swelling mechanism.
06. December 2017
The US FDA and EMA recently issued industry guidance focused on reducing risk associated with medication errors and
improving patient compliance. Recommendations are that varying color, shape, and size between dose strengths of a solid oral medication are useful tools to improve diff erentiation and minimize potential for errors. Additionally, visual differentiation of immediate and modifi ed release dosage forms of the same drug is essential to ensure overall patient safety.
17. September 2017
StarCap 1500 is a unique co-processed mixture of globally accepted excipients, corn starch and pregelatinized starch, designed for use in capsules and tablets. StarCap 1500 is an inert free-flowing compressible excipient with excellent disintegration characteristics. The flow properties of StarCap1500 are ideal for capsule filling applications. StarCap 1500 flows smoothly with minimal dusting or adherence to contact surfaces. This corresponds to a cleaner filling operation with lower variation...
07. August 2017
Jason Teckoe, technical director, Colorcon EMEA, discusses the important considerations when coating oral solid dosage forms.
Jason Teckoe
Jason Teckoe, technical director, Colorcon EMEA
Tablets remain the most common solid oral dosage form for many reasons, including ease of manufacture, convenience for the patient, accurate dose administration and good stability. Good tablet design can be used to provide product differentiation, avoid medication mix-ups and deter counterfeiting.