- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. September 2018
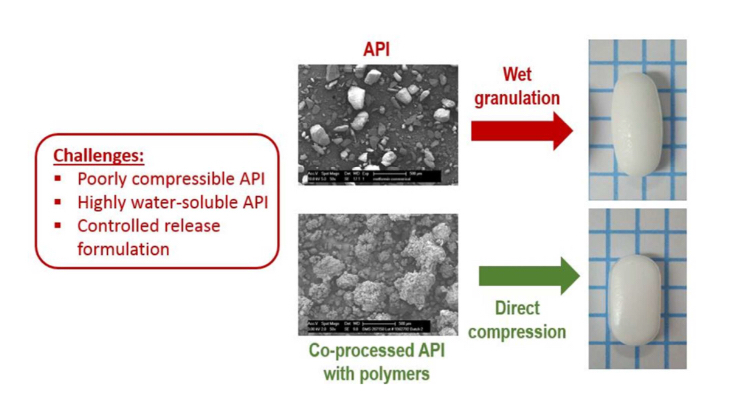
Herein we introduce an innovative process for preparation of directly compressible API and excipient agglomerates for extended release formulation of a highly water soluble drug, demonstrated with metformin HCl. Metformin is poorly compressible and currently employs wet granulation for tablet manufacturing, resulting in long cycle times. We have co-processed metformin HCl with hydroxypropyl methylcellulose (HPMC) and sodium carboxymethlycellulose (NaCMC) in solvent medium to generate...
20. August 2018
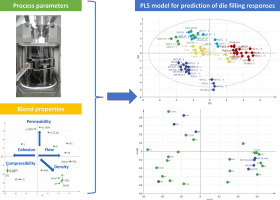
Based on characterization of a wide range of fillers and APIs, thirty divergent blends were composed and subsequently compressed on a rotary tablet press, varying paddle speed and turret speed. The tablet weight variability was determined of 20 grab samples consisting of each 20 tablets. Additionally, the bulk residence time, ejection force, pre-compression displacement, main compression force, die fill fraction and feed frame fill fraction were determined during each run. Multivariate data...
17. August 2018
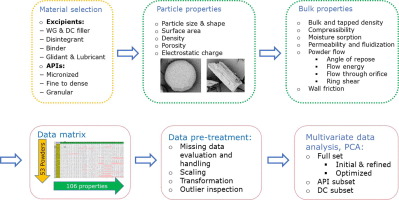
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
17. July 2018
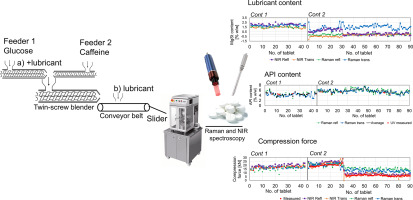
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
24. June 2018
Co-processed excipients may enhance functionality and reduce drawbacks of traditional excipients for the manufacture of tablets on a commercial scale. The following study aimed to characterise a range of co-processed excipients that may prove suitable for dispersible tablet formulations prepared by direct compression. Co-processed excipients were lubricated and compressed into 10.5-mm convex tablets using a Phoenix compaction simulator. Compression profiles were generated by varying the...
07. June 2018
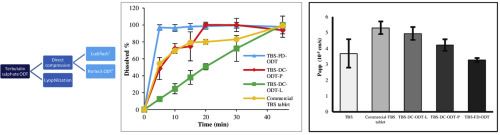
Asthma is a chronic respiratory condition characterized by attacks of spasm in the bronchi of the lungs, causing difficulty in breathing. Oral and inhalation routes are generally used for the treatment of asthma. Terbutaline sulfate (TBS), is a widely used bronchodilator for the treatment of asthma, is available in formulations in the market. However, there is no commercially available orally disintegrating tablets (ODTs) containing TBS. Therefore, this study was aimed to develop and...
09. May 2018
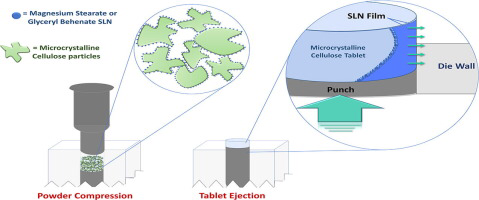
The aim of this study was to develop solid lipid nanoparticles (SLN) and introduce them into a direct compression process to evaluate their lubricant properties. The study consisted of preparing glyceryl behenate SLN (Compritol® 888 ATO) by hot dispersion, and magnesium stearate SLN by a novel nanoprecipitation/ion exchange method. The ejection force was measured for nanosystems and raw materials in a formulation typically used for direct compression. The smallest particle sizes obtained were...
02. May 2018
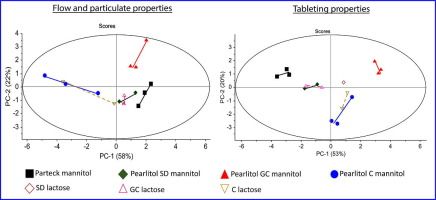
Appropriate selection of excipient grade during tablet formulation development depends on thorough knowledge in their compaction and flow properties. Each chemically unique pharmaceutical excipientis usually available in several commercial grades that are widely different in powder properties, which influence their performance for a specific formulation application. In this work, 11 grades of mannitol were systematically characterized, in terms of their particulate, flow and tableting...
07. April 2018
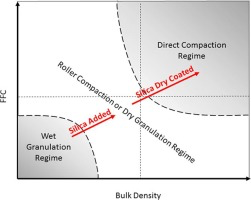
It has been shown that dry coating cohesive active pharmaceutical ingredients (APIs) with nano-silica can improve packing and flow of their blends, facilitating high speed direct compressiontableting. This paper examines the broader scope and generality of previous work by examining three fine APIs; micronized Acetaminophen (mAPAP), coarse Acetaminophen (cAPAP) and micronized Ibuprofen(mIBU), and considers dry coating with both hydrophobic or hydrophilic nano-silica to examine the effect not...
04. April 2018
A full day of lectures on the Basics of
Granulation, Wurster Coating, Tablet
Compression, Tooling and Troubleshooting