- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. May 2018
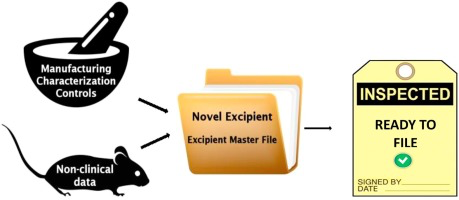
Novel excipients are indispensable in development of modern, advanced drug delivery systems and biotechnology-derived drugs. Although numerous novel excipients are developed for pharmaceutical use, they are not frequently seen in medicinal products due to the strict regulatory requirements and perception that their use makes new product evaluation more complex with risk of delays in the approval process. Regulators regard novel excipients as new substances and whenever new excipient is used in...
07. October 2017
In the context of continuous pharmaceutical oral dosage manufacturing, a control system is essential to ensure that the critical quality attributes (CQAs) are maintained within the regulatory constraints by mitigating variations generated in upstream operations. Such a system is essential to the Quality by Design (QbD) paradigm shift, which can ensure predefined end quality attributes are achieved within an optimal economic and time bracket. In this work, an advanced model predictive control...
09. February 2017
Abstract Computational modelling of twin-screw granulation was conducted by using an artificial neural network (ANN) approach. Various ANN configurations were considered with changing hidden layers, nodes and activation functions to determine the optimum model for the prediction of the process. The neural networks were trained using experimental data obtained for granulation of pure microcrystalline cellulose using a 12 mm twin-screw extruder. The experimental data were obtained for various...
15. November 2016
Excipient regulations, guides, position papers, white papers – here’s how it all fits together! Total Excipient Control (TEC) is the culmination of three decades of IPEC efforts - a "systems approach" to excipient design, safety, manufacturing, and Distribution. Information & Registration
28. May 2016
Orally disintegrating tablets (ODTs) are dosage forms which disintegrate in mouth within seconds without need of water. This type of quality in dosage form can be attained by addition of different varieties of excipients. PharmaburstTM 500 is a co-processed excipient system which allows rapid disintegration and low adhesion to punches. The aim of the present study was to develop and evaluate 25 mg diclofenac sodium ODTs (orodispersible tablets) batches by direct compression method at different...
06. September 2015
Pharmaceutical excipients have different functions within a drug formulation, consequently they can influence the manufacturability and/or performance of medicinal products. Therefore, critical to quality attributes should be kept constant. Sometimes it may be necessary to qualify a second supplier, but its product will not be completely equal to the first supplier product. More
09. June 2015
Highlights • Qualification of entire processes based on CQAs of the final product (excipient). • Use of analytical methods (Raman, X-ray- and laser diffraction) with MVDA. • The value of such combined strategy is to produce a better diagnostics of quality. • Evidence-driven diagnostics of which process delivers a more consistent end-product. • Strategy for a risk-based supplier qualification using an analytical data matrix. Read more
31. May 2015
Key Topics Excipient Design Controls Excipient Safety Excipient Manufacturing Process Control and Distribution Read more and register