- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. August 2018
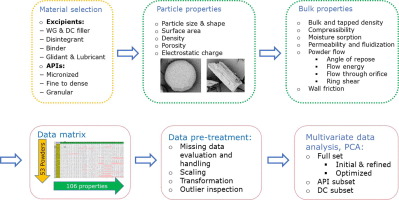
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
15. August 2018
Pharmaceutical tablets contain a variety of active substances and excipients which could be monitored by Raman spectroscopy/microscopy. Raman microscopic spectra are affected not only by the sample composition but also by preparation/measurement conditions. The character and variability of surface morphology (of tablet slices), the appropriate levels of focusing and other adjustable settings of the measurement instrumentation represent important parameters to be considered for obtaining...
18. April 2018
Certificates of analysis (CoAs) are a tangible, and important, manifestation of a manufacturer’s relationship with its suppliers of APIs, excipients, and the other materials used to make drug products. Provided by suppliers to customers as a matter of course, these documents operate at the point where materials, laboratory control systems, and manufacturing intersect.
07. October 2017
Burst drug release is often considered a negative phenomenon resulting in unexpected toxicity or tissue irritation. Optimal release of a highly soluble active pharmaceutical ingredient (API) from hypromellose (HPMC) matrices is technologically impossible; therefore, a combination of polymers is required for burst effect reduction. Promising variant could be seen in combination of HPMC and insoluble Eudragits® as water dispersions. These can be applied only on API/insoluble filler mixture as...
13. April 2017
Abstract Microwave resonance technology (MRT) is known as a process analytical technology (PAT) tool for moisture measurements in fluid-bed granulation. It offers a great potential for wet granulation processes even where the suitability of near-infrared (NIR) spectroscopy is limited, e.g. colored granules, large variations in bulk density. However, previous sensor systems operating around a single resonance frequency showed limitations above approx. 7.5% granule moisture. This paper describes...
20. February 2017
Abstract The overall objective of this work is to understand how excipient characteristics influence the process and product performance for a continuous twin-screw wet granulation process. The knowledge gained through this study is intended to be used for a Quality by Design (QbD)-based formulation design approach and formulation optimization. A total of 9 preferred fillers and 9 preferred binders were selected for this study. The selected fillers and binders were extensively characterized...
29. August 2016
A string of new guidance documents have made it clear that data integrity (DI) remains high on the agenda of regulatory authorities around the world and this has become a global issue. DI in the context of pharma manufacturing has led to a rising number of regulatory enforcement actions citing failures in the recording and review of manufacturing data. In some cases violations have led to written warning letters and even bans on imports from some manufacturing facilities. The extent of draft...
06. September 2015
Pharmaceutical excipients have different functions within a drug formulation, consequently they can influence the manufacturability and/or performance of medicinal products. Therefore, critical to quality attributes should be kept constant. Sometimes it may be necessary to qualify a second supplier, but its product will not be completely equal to the first supplier product. More
09. June 2015
Highlights • Qualification of entire processes based on CQAs of the final product (excipient). • Use of analytical methods (Raman, X-ray- and laser diffraction) with MVDA. • The value of such combined strategy is to produce a better diagnostics of quality. • Evidence-driven diagnostics of which process delivers a more consistent end-product. • Strategy for a risk-based supplier qualification using an analytical data matrix. Read more