- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. August 2018
LIVE WEBCAST Tuesday, October 2, 2018 at 11am EDT, 10am CDT, 4pm BST, 5pm CEST Can't make the live webcast? Register now and view it on-demand after the air date. Event Overview The Inactive Ingredient Database (IID) provides information on inactive ingredients present in FDA-approved drug products and is used by industry as an aid in developing drug products. How can sponsors gain a better understanding of the FDA requirements and avoid lengthy review cycles, unnecessary requests for...
15. January 2017
EudraGMDP is the name for the Union database referred to in article 111(6) of Directive 2001/83/EC and article 80(6) of Directive 2001/82/EC. It contains the following information: Manufacturing and import authorisations Good Manufacturing Practice (GMP) certificates Statements of non-compliance with GMP GMP inspection planning in third countries In addition the following new information is required in the database for the first time in 2013. As data transfer from national systems can be...
18. November 2016
The US FDA has almost cleared a backlog of 4000 excipient formulations in its Inactive Ingredient Database (IID) and wants drugmakers to help ensure ongoing accuracy. The US FDA has almost cleared a backlog of 4000 excipient formulations in its Inactive Ingredient Database (IID) and wants drugmakers to help ensure ongoing accuracy. The IID is intended to be a definitive searchable catalogue of every excipient that has ever been used in a US Food and Drug Administration (FDA) approved drug. The...
25. February 2016
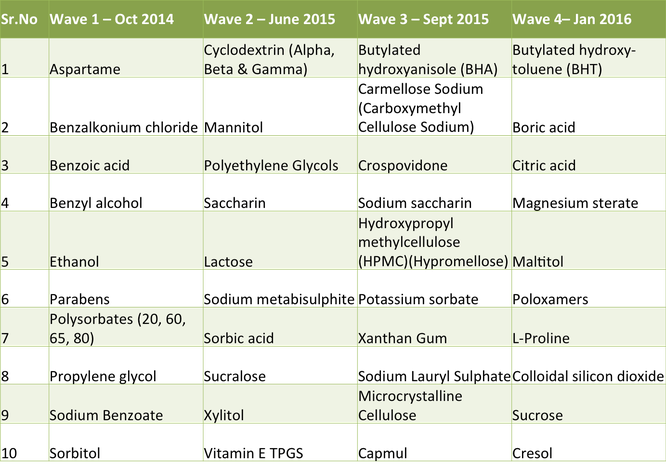
[Alcohol, alpha-Cyclodextrin, Aspartame, Benzalkonium Chloride, Benzoic Acid, Benzyl Alcohol, beta-Cyclodextrin, Butylated Hydroxyanisole, Butylparaben, Carboxymethylcellulose, Carboxymethylcellulose Sodium, Crospovidone, Ethylparaben, gamma-Cyclodextrin, Glyceryl Monocaprylate [Capmul MCM C8], Hypromellose, Lactose, Mannitol, Methylparaben, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 20, Polysorbate 40, Polysorbate 60, Polysorbate 65, Polysorbate 80, Povidone, Propylene...
10. February 2016
The STEP stands for Safety and Toxicity of Excipients for Paediatrics. The STEP database is a user-designed free resource that compiles the safety and toxicity information of excipients that is manually extracted from selected information sources. Three tier quality control procedures are implemented from information retrieval level to entry of the specific data in the database. More
04. October 2015
08. June 2015
The STEP stands for Safety and Toxicity of Excipients for Paediatrics. The Safety and Toxicity of Excipients for Paediatrics (STEP) Database is a user-designed resource developed by European Paediatric Formulation Initiative (EuPFI) and United States Paediatric Formulation Initiative (USPFI) in collaboration for storage and rapid/effortless access to the safety and toxicological data of excipients that is scattered over various sources and presents it in one freely accessible source. It is a...
23. April 2015
Issues related to inaccurate and incomplete information on excipients referenced in the U.S. FDA Inactive Ingredient Database (IID) and FDA policies and guidance related to the review of inactive ingredients in ANDAs continue to create confusion for the pharmaceutical industry. As a result, pharmaceutical companies filing drug applications have encountered longer review cycles, unnecessary requests for additional safety studies/information and/or Refuse to Receive letters from the Agency....