- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
12. September 2018
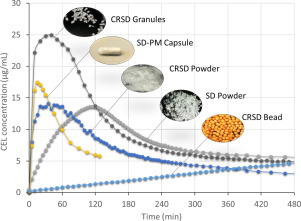
The amorphous solid dispersion (ASD) technique has been employed to formulate poorly-soluble drugs, however, development of solid dosage forms with ASD is challenging due to the high propensity of amorphous drug to precipitate upon dissolution. Thus this work aimed to explore the potential of controlled release amorphous solid dispersion (CRASD) systems using polyvinyl acetate (PVAc) as a release-retarding excipient to mitigate the drug precipitation during dissolution of poorly water-soluble...
02. September 2018
The current study was aimed to develop extended release (ER) pellets formulations containing zaltoprofen as a model drug and cross-linked starch-κ-carrageenan (Sκ-C) hydrogel composite as a binder and extended release polymer. The Sκ-C cross-linked hydrogel composites were prepared using a 32full factorial design approach and characterized by FTIR, DSC, XRD and SEM analysis. The matrix pellets were prepared by extrusion-spheronization technique and characterized production yield, FTIR, DSC,...
17. August 2018
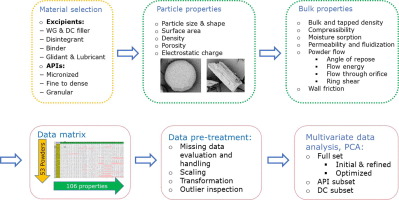
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
08. August 2018
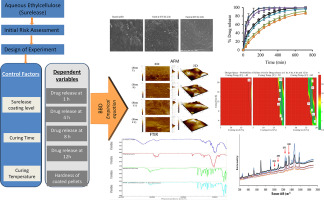
The main purpose of this study was to investigate the influence of ethylcellulose (EC) coating and curing conditions on the quality attributes of pharmaceutical pellets prepared using a quality by design (QbD) approach. The drug release rate of methimazole, a freely soluble model drug, was evaluated extensively together with the mechanical strength and true density of the coated pellets. Moreover, the thermal and spectroscopic properties, as well as the surface characteristics were studied...
21. July 2018
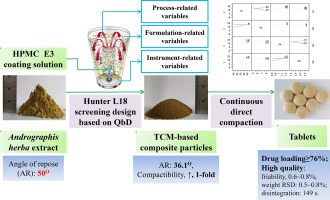
The Andrographis herba extract (AHE), a traditional Chinese medicine (TCM), was developed to directly compactible powders by fluid bed coating with 6% to 12% hydroxypropyl methylcellulose (HPMC). The process-, instrument-, and formulation-related variables of the coating process were simultaneously optimized with the Hunter L18 screening design. Yield (Y1), compactibility (Y2), and angle of repose (Y3) were measured as the responses. The optimized variables were 50 °C for inlet air...
07. July 2018
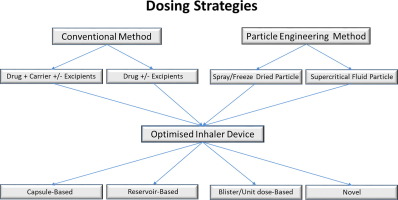
The pulmonary route of administration has been commonly used for local lung conditions such as asthma and chronic obstructive pulmonary disease (COPD). Recently, with the advent of new technologies available for both formulation and device design, molecules usually delivered at high doses, such as antibiotics and insulin to treat cystic fibrosis (CF) and diabetes, respectively, can now be delivered by inhalation as a dry powder. These molecules are generally delivered in milligrams instead of...
07. July 2018
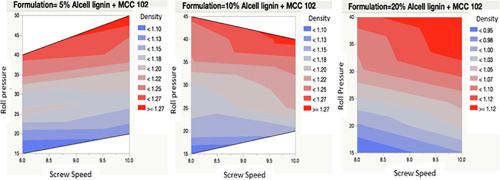
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
02. July 2018
The aim of this study was to develop self-nanoemulsifying drug delivery system (SNEDDS) of bosentan using quality by design (QBD) approach with better bioavailability. The major component of the formulation vis-à-vis lipid (Capmul MCM), surfactant (LABRASOL) and co-surfactant (PEG 600) were selected on the basis of saturation solubility. Mixture of LABRASOL and PEG 600 in the ratio of 1:1 showed better nano emulsifying region as depicted by pseudo ternary phase diagram. The optimum mixture of...
17. May 2018
Current in vitro disintegration methods for polymeric films are qualitative and introduce significant user bias. The goal of these studies is to develop a novel, quantitative disintegration technique which can be used to characterize polymeric films in vitro. Methods A method was developed using a Texture Analyzer instrument to evaluate film disintegration. Solvent-casted, clinically advanced, anti-HIV, vaginal films as well as marketed vaginal films were used throughout these studies. Method...
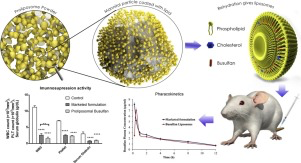
13. November 2017
Parenteral administration of Busulfan (BU) conquers the bioavailability and biovariability related issues of oral BU by maintaining the plasma drug concentration in therapeutic range with minimal fluctuations thereby significantly reducing the side effects. Busulfex® is the only commercially available parenteral formulation of BU composed of organic solvents N, N-dimethylacetamide and polyethylene glycol 400. Since, BU is highly susceptible to hydrolytic degradation; Busulfex® has poor...