- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. August 2018
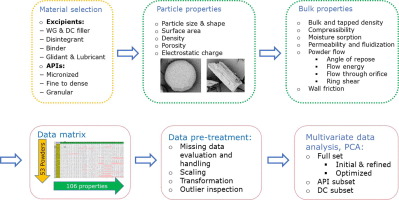
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
17. May 2018
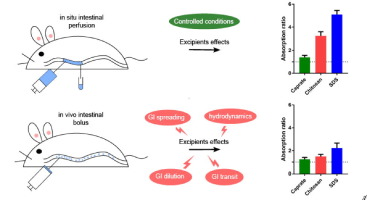
Pharmaceutical excipients that may affect gastrointestinal (GI) drug absorption are called critical pharmaceutical excipients (CPEs), or absorption-modifying excipients (AMEs) if they act by altering the integrity of the intestinal epithelial cell membrane. Some of these excipients increase intestinal permeability, and subsequently the absorption and bioavailability of the drug. This could have implications for both the assessment of bioequivalence and the efficacy of the absorption-enhancing...
27. March 2018
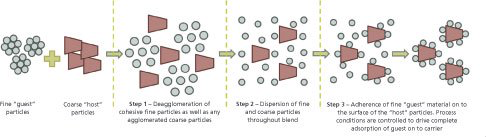
Particle surface modification is becoming a vital strategy in the pharmaceutical industry for “difficult to formulate” APIs. Most surface modification techniques, however, alter innate particle properties either chemically or physically. These surface modification technologies involve the use of elevated temperatures, high pressures, and/or solvents, making these processes highly unsuitable for unstable APIs that are degraded when exposed to such conditions. Aston Particle Technologies...
05. February 2018
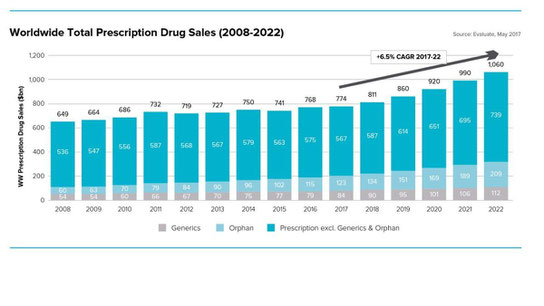
The tenth edition of Evaluate Pharma’s World Preview brings together many of our analyses to provide a top level insight, from the world’s financial markets, into the expected performance of the industry between now and 2022.
22. January 2018
Purdue University researchers and industry leaders are teaming up to transform the freeze-drying process, formally known as lyophilization, used to make everything from lifesaving drugs and biotech products to foods.
A consortium of researchers, industry members from pharmaceutical and food processing sectors, as well as equipment makers and others, are working together at the Advanced Lyophilization Technology Hub, or LyoHub, at Purdue to modernize a process that has not changed over decades.
27. December 2017
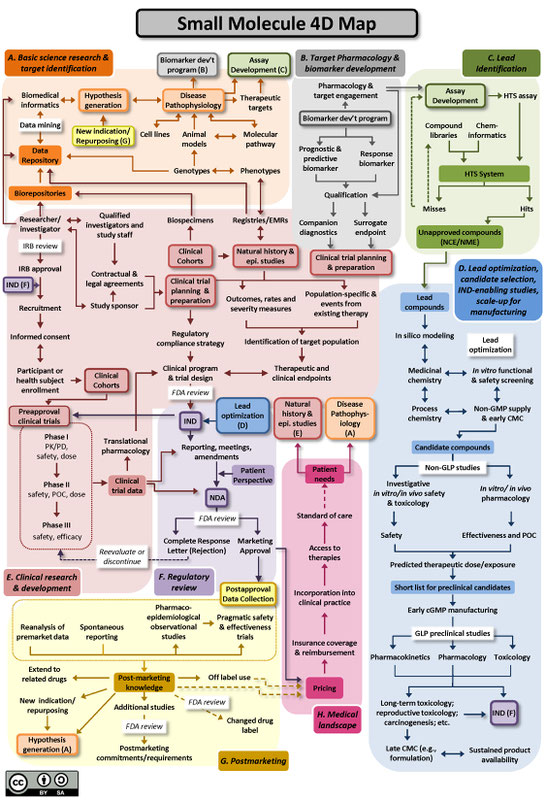
The Drug Discovery, Development and Deployment Maps (4DM) provide dynamic representations of the modern therapeutic development process to more easily identify inefficiencies and integrate efforts to expedite new therapies for patients. The maps provide a common framework for discussing the therapeutic development process and serve as an education tool for those who are new to it.
03. September 2017
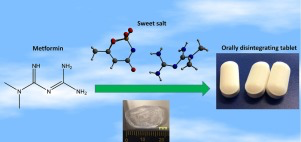
Abstract Salt formation has been intensively used to improve drug properties, including solubility, stability and mechanical properties. A sweet salt of metformin with acesulfame, prepared though an anion exchange reaction, showed superior properties over the commercial hydrochloride salt. These included both remarkable improvement of taste and significant enhancement in tabletability, which is explained by the different crystal structures and lower hardness as measured by nanoindentation. The...
29. April 2017
Abstract Thoughtfully designed early clinical formulations not only meet the needs of the study at hand and inform the development of the commercial product, but can influence the direction of the clinical program as well as provide further guidance to potential backups still in exploratory stages. This chapter focuses on the various types of early clinical formulations, why they are developed, and how the preclinical formulation space helps to guide initial clinical formulation selection....
07. April 2017
Abstract A compound, which is a selective peroxisome proliferator activated receptor (PPAR) agonist, was investigated. The aim of the presented studies was to evaluate the potential of the further development of the compound. Fundamental physicochemical properties and stability of the compound were characterized in solution by liquid chromatography and NMR and in solid-state by various techniques. The drug itself is a poorly soluble lipophilic acid with tendency to form aggregates in solution....
24. March 2017
Abstract Introduction: Most conventional drug delivery systems are not acceptable for pediatric patients as they differ in their developmental status and dosing requirements from other subsets of the population. Technology platforms are required to aid the development of age-appropriate medicines to maximize patient acceptability while maintaining safety, efficacy, accessibility and affordability. Areas covered: The current approaches and novel developments in the field of age-appropriate drug...