- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
10. September 2018
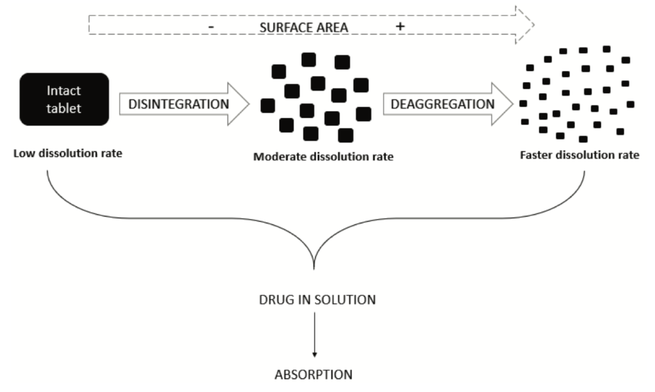
Disintegration is a physical process related to the mechanical breakdown of a tablet or granulate particle into smaller particles. This review investigates disintegration mechanisms, pharmacopeial use of the disintegration test and scientific studies showing its utility and potential as a pharmaceutical performance test. With a proper dosage form understanding and demonstration/justification of the mechanistic details of drug dissolution from a dosage form, dissolution testing might be replaced...
01. November 2017
The FDA guidance on application of the Biopharmaceutics Classification System (BCS) for waiver of in-vivo bioequivalence (BE) studies was issued in August 2000. Since then, this guidance has created world-wide interest among biopharmaceutical scientists in regulatory agencies, academia, and industry towards its implementation and further expansion.