- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
12. September 2018
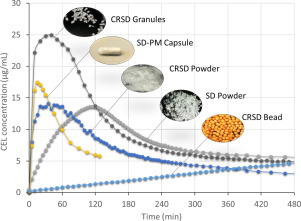
The amorphous solid dispersion (ASD) technique has been employed to formulate poorly-soluble drugs, however, development of solid dosage forms with ASD is challenging due to the high propensity of amorphous drug to precipitate upon dissolution. Thus this work aimed to explore the potential of controlled release amorphous solid dispersion (CRASD) systems using polyvinyl acetate (PVAc) as a release-retarding excipient to mitigate the drug precipitation during dissolution of poorly water-soluble...
17. August 2018
In continuous manufacturing of solid dosage forms, continued assurance of process performance and product quality is based on accurate and consistent flow of solid materials. Acknowledging the multidimensionality of material flow properties is often the first step to explore the material knowledge space.
28. May 2018
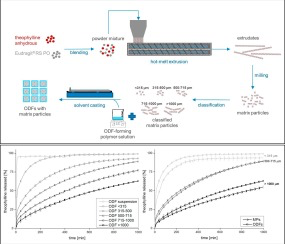
Orodispersible films (ODFs) are an advantageous dosage form to accomplish patient convenience and compliance in oral drug delivery. They provide a number of special application features, such as the ease of administration without water and suitability for patients with swallowing problems. However, this promising dosage form has been limited to immediate release formulations so far. The aim of this study was to develop a thin film produced by solvent casting, which is rapidly disintegrating...
27. May 2018
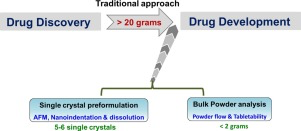
A systematic assessment of key material attributes of active pharmaceutical ingredients (APIs) using minimal material approach is illustrated at the particulate level and bulk level. At a bulk level, flowability improvement of an API through crystal habit manipulation is exemplified. The impact of crystal structures and particulate properties (crystal habit and crystal size) on their mechanical behaviour were addressed by measuring powder tabletability. Bulk powder flow and tabletability were...
01. March 2018
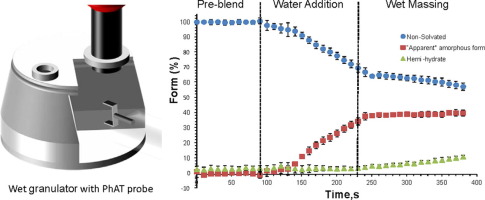
Form changes during drug product processing can be a risk to the final product quality in terms of chemical stability and bioavailability. In this study, online Raman spectroscopy was used to monitor the form changes in real time during high shear wet granulation of Compound A, a highly soluble drug present at a high drug load in an extended release formulation. The effect of water content, temperature, wet massing time and drying technique on the degree of drug transformation were examined.
24. February 2018
Enalapril is an off-patent angiotensin-converting enzyme inhibitor for which no paediatric age-appropriate formulation is commercially available in Europe, and enalapril maleate (EM) orodispersible minitablets (ODMTs) have previously been formulated within the LENA (labelling enalapril from neonates to adolescents) project. In this study, a dilution method has been developed by dispersing the lowest dose strength ODMTs to enable flexible and precise EM dosing during the dose titration phase of...
18. October 2017
According to pediatric experts’ recommendations, an appropriate formulation for children allows minimal dosage and frequency; has minimal impact on lifestyle; contains non-toxic excipients; enables convenient, easy and reliable administration; and is easily produced and commercially viable.
01. October 2017
Recently, we have introduced fibrous dosage forms prepared by the predictable deposition, or 3D-micro-patterning, of a drug-laden fibrous melt on a surface. Such dosage forms enable precisely controlled microstructures and drug release rates, and can be manufactured by an efficient, continuous melt process. However, the applicability of melt-processing to manufacture pharmaceutical dosage forms is limited because the temperatures at which suitable excipients plasticize by melting are greater...
16. September 2017
The choice of excipients constitutes a major part of preformulation and formulation studies during the preparation of pharmaceutical dosage forms. The physical, mechanical, and chemical propertiesofexcipients affect various formulation parameters, such as disintegration, dissolution, and shelf life, and significantly influence the final product. Therefore, several studies have been performedtoevaluate the effect of drug-excipient interactions on the overall formulation. This article reviews the...
20. April 2017
Abstract The purpose of this study is to demonstrate that the flow function (FFc) of pharmaceutical powders, as measured by rotational shear cell, is predominantly governed by cohesion, but not friction coefficients. Driven by an earlier report showing an inverse correlation between FFc and the cohesion divided by the corresponding pre-consolidation stress [Wang et al. 2016. Powder Tech. 294:105-112], we performed analysis on a large dataset containing 1130 measurements from a ring shear...