- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
28. September 2018
Covalently cross-linked and hydrolytically degradable poly(oligoethylene glycol methacrylate) (POEGMA)-based nanogels are fabricated using an all-aqueous self-assembly approach. The nanogels are composed of hydrazide- (POH) and aldehyde-functionalized (POA) POEGMA precursor polymers that exhibit lower critical solution temperature (LCST) behavior in aqueous media and form a covalent, yet degradable, hydrazone linkage upon mixing. By systematically changing the chemistry of the core and...
13. September 2018
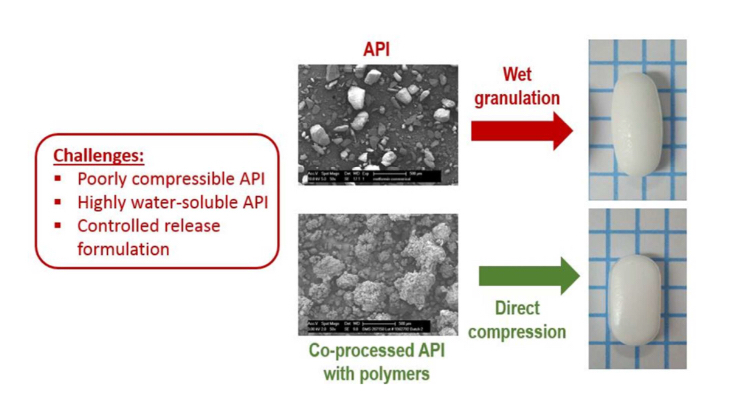
Herein we introduce an innovative process for preparation of directly compressible API and excipient agglomerates for extended release formulation of a highly water soluble drug, demonstrated with metformin HCl. Metformin is poorly compressible and currently employs wet granulation for tablet manufacturing, resulting in long cycle times. We have co-processed metformin HCl with hydroxypropyl methylcellulose (HPMC) and sodium carboxymethlycellulose (NaCMC) in solvent medium to generate...
10. September 2018
Of over 250 entries in 17 categories the finalists have been announced by CPhI. Here is the shortlist of excipients and formulation. Excellence in Pharma: Excipients Adwatis SA – Advanced Water S-100 Evonik Industries AG – EUDRAGIT® FL 30 D-55 Ideal Cures Pvt. Ltd. – INSTA COAT QD Lonza – Capsugel® Colorista™ Capsules Merck – Parteck® MXP Excipient Pharma Excipients International AG – Pharma Excipients Sudeep Pharma Pvt Ltd – Magnesium Stearate EXCiPACT Excellence in Pharma:...
05. September 2018
As Pfanstiehl approaches its 100th anniversary in 2019, the company has launched a new high purity, low endotoxin, and low metal L-Arginine (USP, EP, JP, ChP), manufactured in the United States under strict cGMP, ICH Q7 compliant conditions. This is the first amino acid Pfanstiehl has launched and they have others in development.
25. August 2018
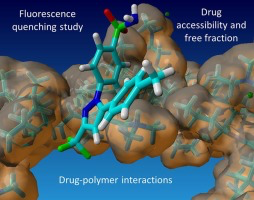
Solid dispersions (SDs) represent an important formulation technique to achieve supersaturation in gastro-intestinal fluids and to enhance absorption of poorly water-soluble drugs. Extensive research was leading to a rather good understanding of SDs in the dry state, whereas the complex interactions in aqueous medium are still challenging to analyze. This paper introduces a fluorescence quenching approach together with size-exclusion chromatography to study drug and polymer interactions that...
21. August 2018
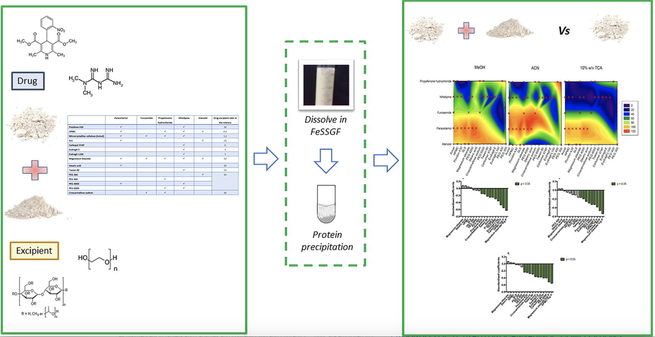
In this study, the influence of the presence of excipients in sample preparation and clean-up steps required prior to drug analysis in milk-based media which simulate the in vivo properties of the fed state stomach was investigated. 15 excipients, normally present in solid dosage forms of five APIs tested (atenolol, paracetamol, furosemide, nifedipine and propafenone hydrochloride) were mixed (one at a time) with the active pharmaceutical ingredient of interest either via vortexing, co-grinding...
30. July 2018
Drug nanoparticles embedded in a dispersant matrix as a secondary phase, i.e., drug-laden nanocomposites, offer a versatile delivery platform for enhancing the dissolution rate and bioavailability of poorly water-soluble drugs. Drug nanoparticles are prepared by top-down, bottom-up, or combinative approaches in the form of nanosuspensions, which are subsequently dried to prepare drug-laden nanocomposites. In this comprehensive review paper, the term “nanocomposites” is used in a broad...
30. July 2018
The number of biologics in the therapeutic development pipeline is increasing including those delivered though inhalation (Morales, 2017; Fathe, 2016). Biologics comprise a broad variety of complex macromolecules with unique physicochemical characteristics. These distinctive characteristics control their pharmacological mechanisms of action, stability, and ultimately affect their processing, formulation, and delivery requirements. This review systematically covers crucial aspects of biologic...
30. July 2018
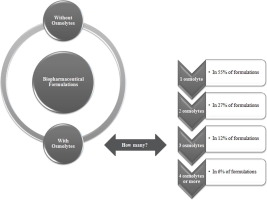
Osmolytes are small organic molecules accumulated by cells in response to environmental stresses. They are represented by amino acids, sugars, polyols, tertiary sulphonium and quaternary ammonium compounds. These molecules present a protective behaviour and favour the equilibrium of macromolecules towards the native form, preventing denaturation and promoting the folding of unfolded proteins. Protein formulations due to their biological character require greater care during the manufacturing...
25. July 2018
Dry Powder Inhaler (DPI) could offer a propellant-free, easy-to-use powder form ensuring better stability than liquid dosae forms. Therefore the development of traditional carrier-based and carrier-free new generation systems is a determinative factor in the field of DPI formulation. The purpose of our research work was to combine these two systems, utilizing their beneficial properties to produce a novel pulmonary drug delivery system containing ciprofloxacin hydrochloride (CIP). Co-spray...