- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. July 2018
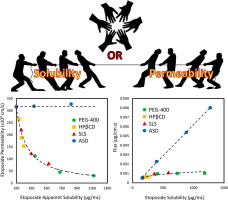
Poor aqueous solubility is a major challenge in today's biopharmaceutics. While solubility-enabling formulations can significantly increase the apparent solubility of the drug, the concomitant effect on the drug's apparent permeability has been largely overlooked. The mathematical equation to describe the membrane permeability of a drug comprises the membrane/aqueous partition coefficient, which in turn is dependent on the drug's apparent solubility in the GI milieu, suggesting that the...
04. July 2018
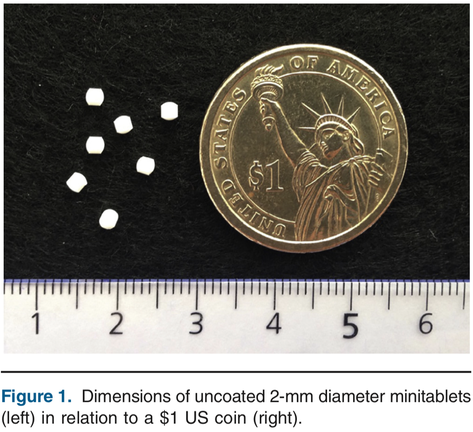
The objective of this study was to assess the acceptability and swallowability of several minitablets when administered as a unit dose compared with an equivalent dose of syrup in children aged 6 months to 5 years. Study design The acceptability and swallowability of multiple drug-free minitablets in comparison with glucose syrup was assessed in 372 children of 2 age groups (186 in age group 1 [6-23 months of age] and 186 in age group 2 [2-5 years of age]) in a randomized, 3-way, single...
26. March 2018
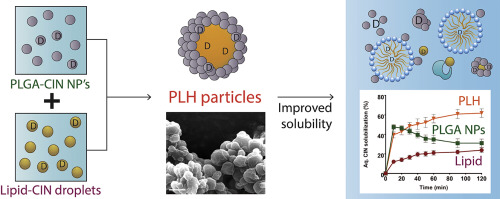
A novel hybrid microparticulate system composed of poly(lactic-co-glycolic) acid (PLGA) nanoparticles and submicron medium-chain triglyceride (MCT) droplets was fabricated to overcome the pH-dependent solubility and precipitation challenges associated with a model poorly water-soluble weak base, cinnarizine (CIN). Molecular CIN was confined within both the lipid and polymer phase of PLGA-lipid hybrid (PLH) and PLGA-lipid-mannitol hybrid (PLMH) particles, which offered significant...
29. November 2017
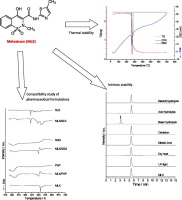
Meloxicam (MLX) is a non-steroidal anti-inflammatory cyclooxygenase (COX) inhibitor that is used to relieve inflammation and pain. MLX has a preferential affinity for COX-2, which is associated with a lower incidence of gastrointestinal side effects. The drug belongs to Class II of the Biopharmaceutical Classification System (BCS) in which dissolution is the limiting step of its bioavailability. In view of this classification, carrying out further studies regarding the compatibility of MLX with...
26. March 2017
Abstract In this work, amorphous paracetamol/Eudragit® formulations for four Eudragit® (polymeric excipients) were prepared by spray drying technique. The simultaneous dissolution kinetics of paracetamol and Eudragit® from these formulations were measured as function of pH in vitro using a rotating disk system (USP II). Paracetamol dissolution mechanisms were analyzed by comparing the dissolution rates of paracetamol and excipient. It was found that a controlled paracetamol dissolution was...
20. February 2017
Abstract Adsorption of lipid-based formulations, which are usually liquid, onto silicas has been extensively investigated in the past decade to convert them into solid dosage forms. There are conflicting reports on the ability of commercially available porous silicas, like Neusilin® US2, to release lipid formulations completely, especially after long-term storage. In this study, the release of a model drug, probucol, from different formulations of medium chain lipids and a surfactant,...
06. February 2017
Abstract Liquid adsorption on solid adsorbent carriers is an emerging technique for oral lipid-based drug delivery systems. The purpose of the current study is to convert liquid into solid self-emulsifying lipid formulations (SELFs) using an inorganic adsorbent Neusilin® grade US2 (NUS2) and investigate in vitro dissolution and digestion performance of the model antipsychotic compound risperidone. Methods The liquid SELFs were designed using various oils, nonionic surfactants and converted...
04. January 2017
Active ingredients in pharmaceuticals di er by their physico-chemical properties and their bioavailabil- ity therefore varies. e most frequently used and most convenient way of administration of medicines is oral, however many drugs are little soluble in water. us they are not su ciently e ective and suitable for such administration. For this reason a system of lipid based formulations (LBF) was developed. Series of formula- tions were prepared and tested in water and biorelevant media. On the...
08. November 2016
Abstract In the current study, we investigated the metoprolol absorption kinetics of an in-house produced oral sustained-release formulation, matrices manufactured via prilling, and two commercially available formulations, ZOK-ZID® (reservoir) and Slow-Lopresor® (matrix) in both New Zealand White rabbits and Beagle dogs, using a population pharmacokinetic analysis approach. The aim of this study was to compare the in vivo pharmacokinetic (PK) profiles of different formulations based on...
15. August 2016
Abstract The saturation solubility of PVP:PZQ physical mixtures (PMs) and solid dispersions (SDs) prepared from ethanol (E/E) or ethanol/water (E/W) by the solvent evaporation method at 1:1, 2:1 and 3:1 ratio (w/w) was determined. The presence of PVP improves the solubility of PZQ (0.31 ± 0.01 mg/mL). A maximum of 1.29 ± 0.03 mg/mL of PZQ in solution was achieved for the 3:1 SD (E/E). The amount of PZQ in solution depends on the amount of polymer and on the preparation method. Solid-state NMR...