- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
03. September 2018
Nowadays, the freeze-drying of liposome dispersions is still necessary to provide a solid dosage form intended for different routes of administration (i.e., parenteral, oral, nasal and/or pulmonary). However, after decades of studies the optimization of process conditions remains still challenging since the freezing and the dehydration destabilize the vesicle organization with the concomitant drug leakage. Starting from the thermal properties of phospholipids, this work reviews the main...
28. August 2018
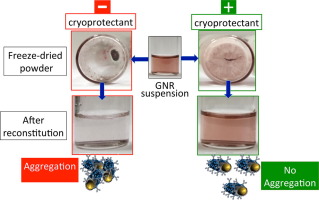
Maintaining colloidal stability of nanoparticles in suspensions is a major challenge. Therefore, freeze-drying (lyophilization) is recently proposed to preserve colloidal stability of nanoparticles through maintaining them in a solid state. However, freeze-drying would itself induce nanoparticle aggregation unless proper formulation with a careful selection of cryoprotectants is considered. Herein, we evaluate the colloidal stability of gold nanorods (GNRs) conjugated with a rituximab as a...
05. April 2018
The dehydration of biopharmaceutical products through drying provides numerous benefits, including ease of handling and storage, reduction in transportation costs, and improved stability. Typically, the drying of biotherapeutics is accomplished through freeze-drying, however, the removal of water by lyophilization possesses several drawbacks, including lengthy drying times, low energy efficiency, and the high cost of purchasing and maintaining the equipment. Furthermore, freeze- drying is a...
13. March 2018
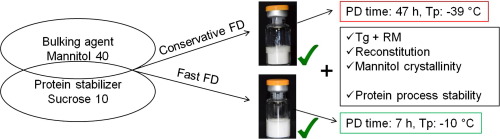
Mannitol/sucrose formulations are employed to generate lyophilizates for biopharmaceuticals with an elegant cake appearance. The aim of this study was to dry protein/mannitol/sucrose formulations as fast as possible without loss of cake appearance and protein stability. Glycerol was included as potential additional protein stabilizer. Three proteins (lysozyme and two monoclonal antibodies) at low and high concentration were analyzed comparing fast with conservative freeze-drying.
03. March 2018
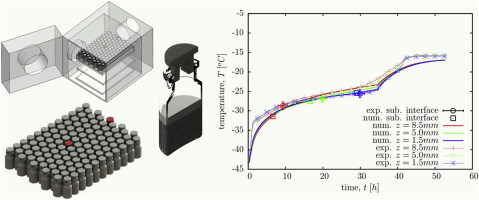
The paper reports on the development of a numerical model for the simulation of a lyophilization process in a vial. Experimental analysis is presented of lyophilization dynamics inside a single vial in a laboratory scale lyophilizer. The problems of lyophilization modelling of a mannitol water solution are covered in detail. The effects of the small scale of the laboratory device with respect to a correct definition of boundary conditions for the numerical simulations are described, especially t
21. January 2018
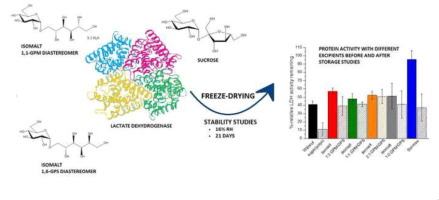
The purpose of this research was to study isomalt as a protein-stabilizing excipient with lactate dehydrogenase (LDH) during freeze-drying and subsequent storage and compare it to sucrose, a standard freeze-drying excipient. Four different diastereomer mixtures of isomalt were studied. The stability of the protein was studied with a spectrophotometric enzyme activity test and circular dichroism after freeze-drying and after 21 days of storage at 16% RH.
03. December 2017
Successful development of marketable freeze-dried protein formulations requires adequate stabilization of the active biopharmaceutical ingredient. The choice of a stabilizer must therefore be based on sound knowledge of the physical and chemical properties of the excipients and specific needs of the protein component.
14. September 2017
Solid dispersions of leflunomide with polymers are prepared by several methods.
Improved dissolution rate of leflunomide by solid dispersion approach is estimated.
Influence of the preparation method and polymer nature on the dissolution is revealed.
Solid dispersions for immediate and sustained release are proposed.
11. September 2017
Solid dispersions of leflunomide with polymers are prepared by several methods.
Improved dissolution rate of leflunomide by solid dispersion approach is estimated.
Influence of the preparation method and polymer nature on the dissolution is revealed.
Solid dispersions for immediate and sustained release are proposed.
27. December 2016
Abstract This study aimed to evaluate aminoclay (3-aminopropyl-functionalized magnesium phyllosilicate) as an effective protectant for the stabilization of protein formulation in freeze-drying. Bovine serum albumin (BSA), as a model protein, was freeze-dried with aminoclay at various concentrations, and the effects of aminoclay on the structural stability of proteins were compared with those of the conventional stabilizers. The structural characteristics of the protein were determined by size...