- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. August 2018
The aim of this study is to determine favorable process conditions for the coating of placebo tablets. Tablets made of microcrystalline cellulose are coated with hydroxypropyl cellulose polymer and Advantia™ Prime polymeric mixture film in lab-scale fluid-bed environment with a Wurster tube. In order to determine favorable process conditions (concentration, Wurster tube position, inlet air temperature, and atomization pressure), evaluation factors expressing process efficiency were...
20. July 2018
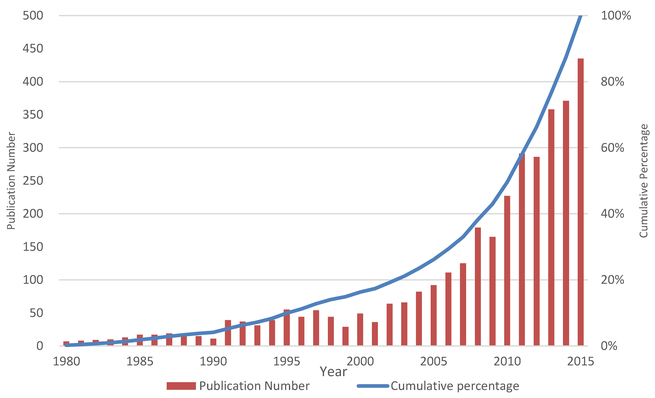
Solid dispersions are an effective formulation technique to improve the solubility, dissolution rate, and bioavailability of water-insoluble drugs for oral delivery. In the last 15 years, increased attention was focused on this technology. There were 23 marketed drugs prepared by solid dispersion techniques. Objective: This study aimed to report the big picture of solid dispersion research from 1980 to 2015. Method: Scientific knowledge mapping tools were used for the qualitative and the...
19. July 2018
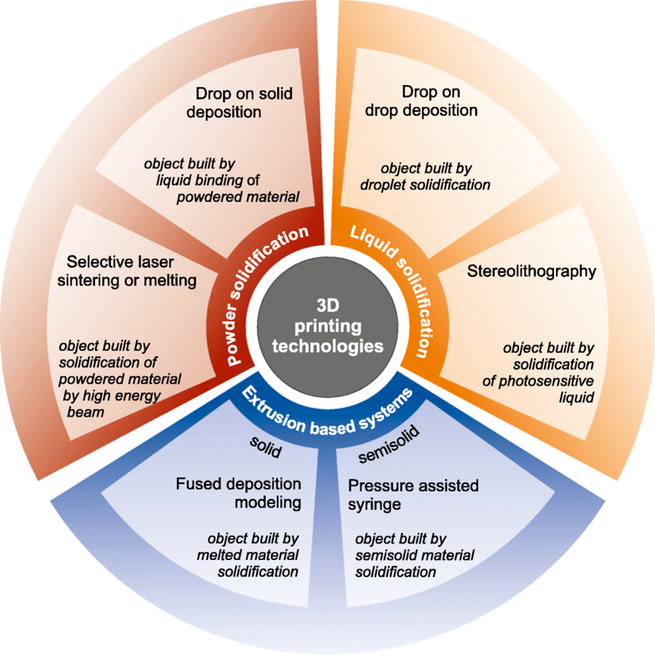
Growing demand for customized pharmaceutics and medical devices makes the impact of additive manufacturing increased rapidly in recent years. The 3D printing has become one of the most revolutionary and powerful tool serving as a technology of precise manufacturing of individually developed dosage forms, tissue engineering and disease modeling.
10. March 2018
Mucoadhesive tablets for administration in buccal mucosa are unconventional formulations with many technological attractions. However there is no standardization of information for its formulation. The present article aims to evaluate, by means of a systematic review with meta-analysis, the data related to the final quality of this technology.
02. March 2018
The oral cavity is frequently used to administer pharmaceutical drug products. This route of administration is seen as the most accessible for the majority of patients and supports an independent therapy management. For current oral dosage forms under development, the prediction of their unintended mucoadhesive properties and esophageal transit profiles would contribute for future administration safety, as concerns regarding unintended adhesion of solid oral dosage forms (SODF) during oro-esopha
15. February 2018
The poor oral bioavailability of many active pharmaceutical ingredients (APIs) resulting from low solubility is one of the important challenges in pharmaceutical technology. Over the last two decades the number of relatively insoluble drugs has grown steadily. Nowadays it is estimated that approximately 70% of new drug candidates are characterized by poor solubility.
12. February 2018
Since decades, granulation is operational as a critical size enlargement process for powder agglomeration in tablet manufacturing. Dry granulation, melt granulation and wet granulation are some of the most common techniques utilized for granulation in the pharmaceutical industry.
19. January 2018
The authors studied six different grades of HPC to determine how they performed in high-shear and fluid-bed granulation.
15. January 2018
In line with the increasing demand for sustainable packaging materials, this contribution aimed to investigate the film-forming properties of hydroxypropyl methylcellulose (HPMC) to correlate its chemical structure with film properties. The roles played by substitution degree (SD) and molecular weight (Mw) on the mechanical and water barrier properties of HPMC films were elucidated.
09. January 2018
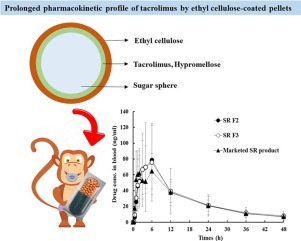
A novel once-a-day sustained-release (SR) system of tacrolimus (FK506), a poorly water-soluble immunosuppressive agent, was designed employing ethyl cellulose (EC) polymer as release retardant. Drug (5 mg) was layered onto sugar spheres (518.3 mg) with hypromellose (5 mg), to transform the drug from a crystalline to an amorphous form.