- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. August 2018
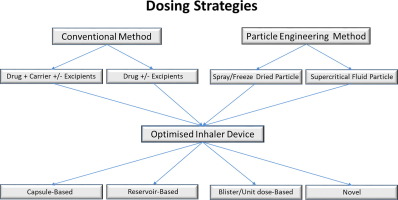
The aim of this study was to develop roflumilast dry powder inhaler (DPI) formulations by spray drying using hydroxypropyl-β-cyclodextrin (HPβCD) and to determine their suitability for pulmonary delivery. Different feed solution concentrations, solvent systems and spray drying parameters were used to obtain the formulations which were characterized using X-ray powder diffraction, thermal analysis, scanning electron microscopy, particle size distribution, bulk and tapped density, specific...
07. July 2018
The pulmonary route of administration has been commonly used for local lung conditions such as asthma and chronic obstructive pulmonary disease (COPD). Recently, with the advent of new technologies available for both formulation and device design, molecules usually delivered at high doses, such as antibiotics and insulin to treat cystic fibrosis (CF) and diabetes, respectively, can now be delivered by inhalation as a dry powder. These molecules are generally delivered in milligrams instead of...
15. April 2018
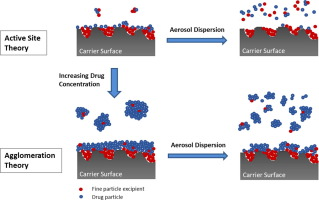
This study was performed to investigate how increasing the active pharmaceutical ingredient (API) content within a formulation affects the dispersion of particles and the aerosol performance efficiency of a carrier based dry powder inhalable (DPI) formulation, using a custom dry powder inhaler (DPI) development rig. Methods Five formulations with varying concentrations of API beclomethasone dipropionate (BDP) between 1% and 30% (w/w) were formulated as a multi-component carrier system...
20. December 2017
The potential of the force control agent (FCA) magnesium stearate (MgSt) to enhance the aerosol performance of lactose-based dry powder inhaled (DPI) formulations was investigated in this study. The excipient blends were investigated with analytical techniques including time-of-flight secondary ion mass spectrometry (ToF-SIMS) and Single Particle Aerosol Mass Spectrometry (SPAMS) and particle size, morphology and surface properties were evaluated.
06. December 2017
Inhaled steroids are currently the most widely used and recommended medicines for the management of persistent asthma because of their high local and anti-inflammatory efficacy.1 There are three main presentations of the drug: aerosol spray (hydrofluoroalkane - HFA - as a propellant), liquid solution and dry powder inhaler.
06. November 2017
Many efforts have been made in the past to understand the function of lactose fines which are given as a ternary component to carrier-based dry powder inhaler formulations. It is undisputed that fines can significantly improve the performance of such formulations, but choosing the right amount of fines is a crucial point, because too high concentrations can have negative effects on the dispersion performance
21. April 2017
Abstract The potential of fine excipient materials to improve the performance of carrier-based dry powder inhalation mixtures is well acknowledged. The mechanisms underlying this potential are, however, open to question till date. Elaborate understanding of these mechanisms is a requisite for rational rather than empirical development of ternary dry powder inhalation mixtures. While effects of fine excipient materials on drug adhesion to and detachment from surfaces of carrier particle have...
24. March 2017
Abstract Introduction: Early dry powder inhalers (DPIs) were designed for low drug doses in asthma and COPD therapy. Nearly all concepts contained carrier-based formulations and lacked efficient dispersion principles. Therefore, particle engineering and powder processing are increasingly applied to achieve acceptable lung deposition with these poorly designed inhalers. Areas covered: The consequences of the choices made for early DPI development with respect of efficacy, production costs and...
09. February 2017
Abstract The effects of propellant type, cosolvent content, and air humidity on the morphology and solid phase of the particles produced from solution pressurized metered dose inhalers containing the corticosteroid beclomethasone dipropionate were investigated. The active ingredient was dissolved in the HFA propellants 134a and 227ea with varying levels of the cosolvent ethanol and filled into pressurized metered dose inhalers. Inhalers were actuated into an evaporation chamber under controlled...
29. January 2017
Background Inhaled medication is the cornerstone of the pharmacological treatment for patients with asthma and chronic obstructive pulmonary disease (COPD). Several inhaler devices exist, and each device has specific characteristics to achieve the optimal inhalation of drugs. The correct use of inhaler devices is not granted and patients may incur in mistakes when using pressurized metered-dose inhalers (pMDIs) or dry-powder inhaler (DPIs). The incorrect use of inhaler devices can lead to a...