- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. January 2018
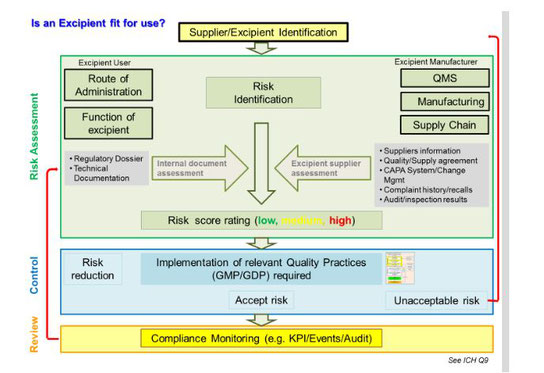
Excipients serve a critical role in the production of final dosage forms for drug products and biologics. They facilitate the manufacturing process (e.g., anticaking agents) and protect, support, and enhance stability. They may also improve bioavailability. In addition, excipients help maintain the safety, or function, of the product during storage and use.
No longer characterized as inert accompaniments to an active pharmaceutical ingredient (API), excipients are the target of an intensified p
31. January 2018
Multiple brands of the same active ingredient may be available for the same strength, administration route and dose form. Generic brands needs to demonstrate bioequivalence to the originator brand, but the appearance of the generic and originator brands are not required to match.
31. January 2018
Lactose intolerance is exceedingly common, reportedly affecting up to 70% of the world’s population, leading to both abdominal and systemic symptoms. Current treatment focuses predominantly on restricting dietary consumption of lactose. Given lactose is one of the most commonly used excipients in the pharmaceutical industry, consideration must be given to the lactose content and therefore safety of pharmaceutical preparations prescribed for patients with lactose intolerance.
30. January 2018
Many drugs are bitter and overcoming this bitter taste is a major barrier in developing a successful product, especially for pediatric patients. Approaches to mask taste include changing taste perception, creating a physical barrier to separate the drug from interacting with taste buds, and changing drug solubility.
30. January 2018
Continuous Manufacturing Technology continues to gain importance in pharmaceutical manufacturing.
Although traditional processes like direct compression, roller compaction, or wet granulation are used within the continuous lines, the requirement for ingredients may differ from traditional batch processing.
29. January 2018
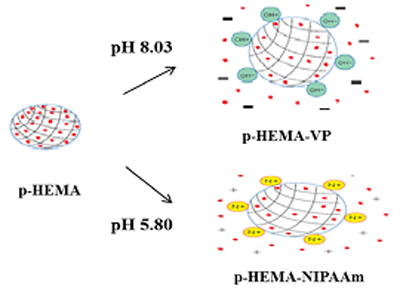
In this study, we evaluated contact lenses as drug delivery media and the effect of tear physiological pH on drug release behavior. Anionic or cationic hydroxyethyl methacrylate (HEMA) lenses were polymerized with either vinylpyrrolidone (VP) or N-isopropylacrylamide (NIPAAm). The pH of artificial tears was prepared as between 5.8 and 8.35. One of common treatments, hydroxypropyl methylcellulose (HPMC), to dry eye disease was used as a test drug.
29. January 2018
The head of the U.S. Food and Drug Administration on Thursday said it is preparing a new, more restrictive policy targeting what drugs compounding pharmacies can produce that do not go through the agency's approval process.
26. January 2018
Cyclodextrins (CDs) are cyclic oligosaccharides which are able to form inclusion complexes with a variety of hydrophobic guest molecules, and are mainly utilized in the pharmaceutical field to enhance the water solubility and dissolution rate of poorly-soluble drugs, to mask unpleasant taste or odor, improve the stability of the included host molecules from hydrolysis or enzymatic degradation in the gastric environment, and reduce local drug irritation phenomena...
26. January 2018
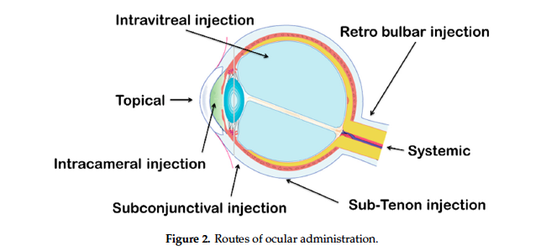
The last fifty years, ophthalmic drug delivery research has made much progress,
challenging scientists about the advantages and limitations of this drug delivery approach. Topical eye
drops are the most commonly used formulation in ocular drug delivery. Despite the good tolerance
for patients, this topical administration is only focus on the anterior ocular diseases and had a high
precorneal loss of drugs due to the tears production and ocular barriers. Antibiotics are popularly
used in solution
26. January 2018
The understanding of amorphous solid dispersions has grown significantly in the past decade. This is evident from the number of approved commercial amorphous solid dispersion products. While amorphous formulation is considered an enabling technology, it has become the norm for formulating poorly soluble compounds.