- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
27. June 2018
In this volume, the authors discuss the many significant challenges currently faced in biotechnology dosage form development, providing guidance, shared experience and thoughtful reflection on how best to address these potential concerns. As the field of therapeutic recombinant therapeutic proteins enters its fourth decade and the market for biopharmaceuticals becomes increasingly competitive, companies are increasingly dedicating resources to develop innovative biopharmaceuticals to address...
25. June 2018
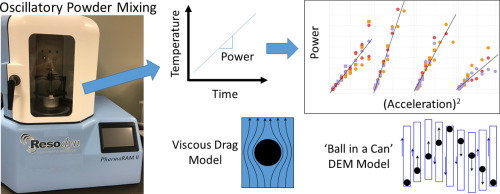
The blending of powders is a critical step in pharmaceutical drug product manufacturing as it ensures that each dosage unit will contain the same intended fraction of active pharmaceutical ingredient (API). Under-blending thus poses a significant risk to patients where overdose or underdose can occur. Similarly, over-blending can lead to manufacturing issues such as reduced tablet tensile strengths as any included lubricants are dispersed further and further with additional blending. For these...
24. June 2018
Co-processed excipients may enhance functionality and reduce drawbacks of traditional excipients for the manufacture of tablets on a commercial scale. The following study aimed to characterise a range of co-processed excipients that may prove suitable for dispersible tablet formulations prepared by direct compression. Co-processed excipients were lubricated and compressed into 10.5-mm convex tablets using a Phoenix compaction simulator. Compression profiles were generated by varying the...
21. June 2018
Reverse Engineering or Deformulation is often used to investigate the composition of a competitor’s product, but is also a powerful tool to examine or monitor formulations during development, scale-up or manufacturing; to reveal and compare hidden excipient properties, like the presence of potential reactive impurities or functional groups, degradation products and related substances, but also molecular weight distributions, degree of substitution, substituent distribution, monomer ratio and...
21. June 2018
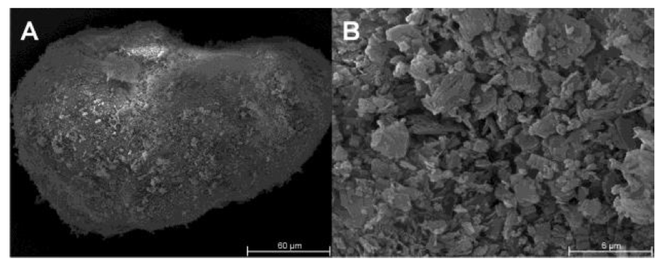
Tricalcium citrate (TCC) was characterized as a tableting excipient for direct compression (DC) and dry granulation (DG). SIGNIFICANCE: Brittle materials usually lead to tablets of inferior mechanical strength compared to plastic deforming materials. A brittle material exhibiting a high tabletability with the ability to retain that behaviour during recompression would represent a valuable alternative to the commonly used microcrystalline cellulose (MCC). METHODS: Tablets of TCC and other common...
20. June 2018
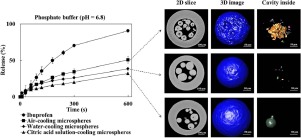
The microsphere was a primary particulate system for taste-masking with unique structural features defined by production process. In this article, ibuprofen lipid microspheres of octadecanol and glycerin monostearate were prepared to mask the undesirable taste of ibuprofen via three kinds of spray congealing processes, namely, air-cooling, water-cooling and citric acid solution-cooling. The stereoscopic and internal structures of ibuprofen microspheres were quantitatively analyzed by...
16. June 2018
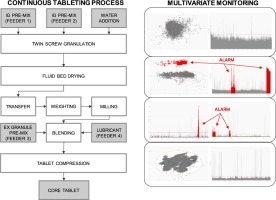
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of...
15. June 2018
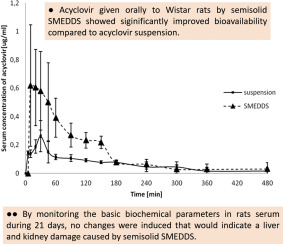
Semisolid self-microemulsifying drug delivery system (SMEDDS) with optimized drugloading capacity, stability, dispersibility in aqueous media and invitro drug release profile, was evaluated in vivo regarding effects on pharmacokinetics of acyclovir, an antiviral with low bioavailability (BA) and short half-life (t1/2). Additional goal of this study was evaluation of safety of this semisolid SMEDDS consisted of medium chain length triglycerides (oil) (10% w/w), macrogolglycerol hydroxystearate...
14. June 2018
The brand new Glatt TwinPro® is absolutely revolutionary. It combines two previously single batch processes into one single process: High Shear Granulation and Fluidized Bed Drying. Critical aspects like product Transfer and wet sieving are eliminated, process time is enormously shortend. Glatt TwinPro® - Innovation by Experience. TwinPro® is the winner of the Achema 2018 Innovation Award - Category Pharmaceutical Technologies.
14. June 2018
The pharmaceutical industry has found new applications for the use of continuous processing for the manufacture of new therapies currently in development. The transformation has been encouraged by regulatory bodies as well as driven by cost reduction, decreased development cycles, access to new chemistries not practical in batch, improved safety, flexible manufacturing platforms, and improved product quality assurance. The transformation from batch to continuous manufacturing processing is the...