- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. July 2018
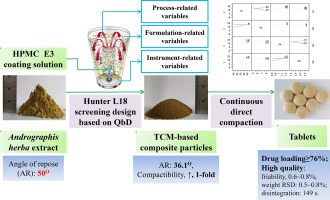
The Andrographis herba extract (AHE), a traditional Chinese medicine (TCM), was developed to directly compactible powders by fluid bed coating with 6% to 12% hydroxypropyl methylcellulose (HPMC). The process-, instrument-, and formulation-related variables of the coating process were simultaneously optimized with the Hunter L18 screening design. Yield (Y1), compactibility (Y2), and angle of repose (Y3) were measured as the responses. The optimized variables were 50 °C for inlet air...
07. June 2018
3D printing evolved as a promising technique to improve individualization of drug therapy. In particular, when printing sustained release solid dosage forms, as for instance implants, inserts, and also tablets, estimation of the drug release profile in vivo is necessary. In most cases, corresponding analyses cannot be performed at hospital or community pharmacies. Therefore, the present study aimed to develop a sustained release drug delivery system produced via 3D printing, which allows dose...
24. February 2018
Enalapril is an off-patent angiotensin-converting enzyme inhibitor for which no paediatric age-appropriate formulation is commercially available in Europe, and enalapril maleate (EM) orodispersible minitablets (ODMTs) have previously been formulated within the LENA (labelling enalapril from neonates to adolescents) project. In this study, a dilution method has been developed by dispersing the lowest dose strength ODMTs to enable flexible and precise EM dosing during the dose titration phase of...
06. November 2017
Jellies for oral administration are dosage forms that contain water, as stipulated in the Japanese Phar- macopeia, and heat is generally applied to the jellies during the manufacturing process. Therefore, it is difficult to formulate drugs that may be affected adversely by water and/or heat.
29. September 2017
An updated version of the Therapeutic Goods (Permissible Ingredients) Determination was registered on the Federal Register of Legislation (FRL) in September 2017. The updated determination is titled the Therapeutic Goods (Permissible Ingredients) Determination No. 4 of 2017.
09. August 2017
he use of unlicensed and off-label medicines in children is widespread and has raised an increasing concern over the last years. The majority of medicines taken by children are extemporaneously compounded by pharmacist, and there is a lack of information regarding bioavailability, suitability and stability. These formulations must be prepared from pure active substance and not from commercially available dosage forms. The development of paediatric formulations, particularly those suitable for ve
30. March 2017
Abstract Chitin is one of the most abundant natural polymers in the world and is used for the production of chitosan by deacetylation. Chitosan is nontoxic and biodegradable and, therefore, can be used as a biomaterial and for the construction of drug delivery systems. Nevertheless, the poor solubility of chitosan in neutral or alkalinized media has restricted its applications in the pharmaceutical and biomedical fields. Chitosan can be easily carboxymethylated to improve its solubility in...
10. October 2016
Abstract A lack of evidence to guide the design of age-appropriate and acceptable dosage forms has been a longstanding knowledge gap in paediatric formulation development. The Children’s Acceptability of Oral Formulations (CALF) study captured end-user perceptions and practices with a focus on solid oral dosage forms, namely tablets, capsules, chewables, orodispersibles, multiparticulates (administered with food) and mini-tablets (administered directly into the mouth). A rigorous development...
24. August 2016
Since the introduction of pediatric regulations in the USA (Pediatric Research Equity Act and Best Pharmaceuticals for Children Act) and Europe, there has been a huge increase in the number of medicines that have information about their use for children. However, despite the expansion of research in the development of pediatric medicinal products, there is still an unmet need for off-patent medicines for children. Incentives in the form of funding support and data protection have not markedly...
25. July 2016
Abstract The feasibility of a colorimetric technique was investigated in CIELAB color space as an analytical quality control method for content uniformity of printed orodispersible pediatric delivery systems. Inkjet printing was utilized to fabricate orodispersibe film formulations containing propranolol hydrochloride in a colored ink base using three different edible substrates. A thin sweetener coating layer of saccharin was successfully included in the final dosage forms for palatability...