- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. October 2017
Filling a dosator nozzle moving into a powder bed was investigated using the Discrete Element Method (DEM). Various particle diameters and contact properties were modeled. The simulations qualitatively showed the influence of powder properties on the amount of dosed powder.
16. September 2017
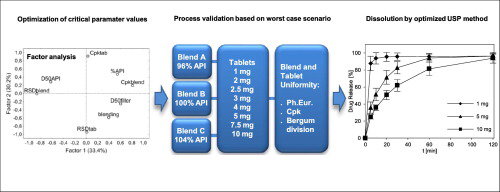
Abstract Warfarin is intensively discussed drug with narrow therapeutic range. There have been cases of bleeding attributed to varying content or altered quality of the active substance. Factor analysis is useful for finding suitable technological parameters leading to high content uniformity of tablets containing low amount of active substance. The composition of tabletting blend and technological procedure were set with respect to factor analysis of previously published results. The...
25. March 2017
Aim: To increase the solubility of azithromycin by formulating solid dispersion (SD) and then SD tablets (SDT) were prepared from the best formulation of SDs. Material and Methods: SDs were prepared using polyethylene glycol 6000 and β-cyclodextrin (β-CD) by solvent evaporation method. To investigate drug-excipient interaction and for selection of suitable excipient for formulation differential scanning calorimetry study was done, and each excipient was selected for formulation development...
11. January 2017
Abstract With the implementation of quality by design (QbD), critical attributes of raw material (drug substance and excipients) are of significantly importance in pharmaceutical manufacturing process. It is desirable for the quality control of critical material attributes (CMAs) of excipients to ensure the quality of end product. This paper explored the feasibility of an at-line method for the quantitative analysis of hydroxypropoxy group in hydroxypropyl methylcellulose (HPMC) with near...
22. November 2016
Abstract The parameters affecting the recovery of pharmaceutical residues from the surface of stainless steel coupons for quantitative cleaning verification method development have been studied, including active pharmaceutical ingredient (API) level, spiking procedure, API/excipient ratio, analyst-to-analyst variability, inter-day variability, and cleaning procedure of the coupons. The lack of a well-defined procedure that consistently cleaned coupon surface was identified as the major...
16. November 2016
Context: Sustained release drug delivery systems are more preferred than the conventional drug delivery systems due to its enhanced bioavailability and patient compliance. Earlier studies reported on glibenclamide (GBCM) were not clear and hence, the step has been taken to explore the sustained release drug delivery system of GBCM. Aims: To evaluate the sustained release microspheres obtained of GBCM. Methods: Microspheres were prepared by ionic gelation method using the polymers like Eudragit...
04. November 2016
Abstract Nanofibers combined with an antimicrobial represent a powerful strategy for treatment of various infections. Local infections usually have a low fluid volume available for drug release, whereas pharmacopoeian dissolution tests include a much larger receptor volume. Therefore, the development of novel drug-release methods that more closely resemble the in-vivo conditions is necessary. We first developed novel biocompatible and biodegradable chitosan/polyethylene oxide nanofibers using...
04. September 2016
Abstract The die filling process within a lab-scale rotary tablet press is simulated using the Discrete Element Method (DEM). The particle motion is captured as particles travel from the gravity feeder consisting of a chute connected to the shoe represented by a rectangular box to the dies/cavities of the turret. The particles fill the dies mostly due to gravity. The process conditions such as die table speed, die diameter as well as the material characteristics e.g. cohesion, coefficient of...
04. August 2016
Abstract Orodispersible films possess a great potential as a versatile platform for nanoparticle-loaded oral dosage forms. In this case, poorly water-soluble organic materials were ground in a stirred media mill and embedded into a polymer matrix. The aim of this study was the shortening of this manufacturing process by the integration of several process steps into a stirred media mill without facing disadvantages regarding the film quality. Furthermore, this process integration is time...
25. July 2016
Present research was aimed with the objective of formulation of Zolpidem fast dissolving oral films, for rapid dissolution of drug and absorption, which may produce the rapid on set off action. The fast dissolving oral films were prepared by solvent casting method using various polymers like HPMC E5, HPMC E15, HPMC K15, microcrystalline cellulose and poly vinyl alcohol. Glycerol used as plasticizer. Prepared films were evaluated for various physicochemical parameters like film thickness,...