- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
23. April 2018
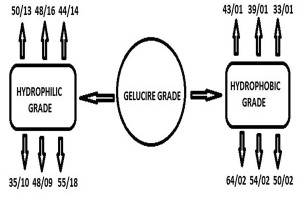
Poly ethylene glycol (PEG) ester surfactants are synthesized by reacting polyethylene glycol with fatty acid. The polyethylene glycol comprises the hydrophilic part of the surfactant and the fatty acid is the lipophilic part. By varying the molecular weight of the PEG and the fatty acid, surfactants covering wide range of hydrophilic lipophilic balance (HLB) values can be produced. Gelucire is the family of vehicle derived from mixtures of mono, di and triglycerides with PEG esters of fatty...
31. December 2017
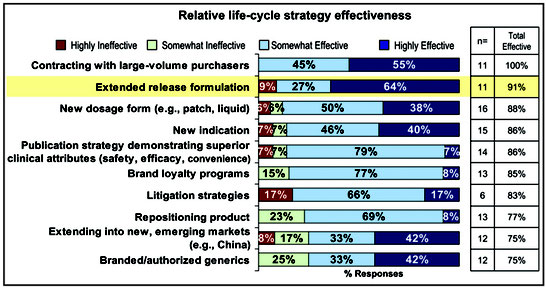
The FDA clarified the regulatory thicket surrounding the 505(b)(2) approval pathway for drug formulations just over a decade ago. In the intervening years, this pathway has become an increasingly popular way for drug makers to commercialize products. The 505(b)(2) pathway allows companies to make modest changes to an already approved drug and get continued market exclusivity for from three to as many as seven years.
10. November 2017
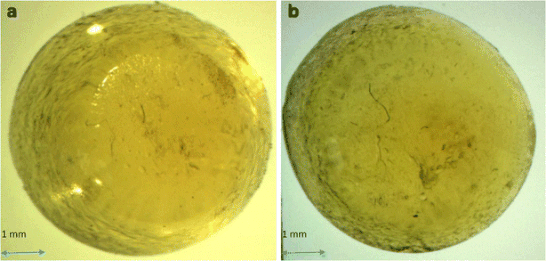
The presented work describes the formulation and characterization of modified release glassy solid dosage forms (GSDFs) containing an amorphous nifedipine, as a model BCS (Biopharmaceutical Classification System) class II drug.
19. July 2017
Nutraceuticals provide an additional health or medicinal benefit besides their nutritional value and are therefore marketed for the prevention and treatment of certain conditions. Nutraceuticals contain natural ingredients, usually presented in the form of functional foods or as dietary supplements.