- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
18. August 2018
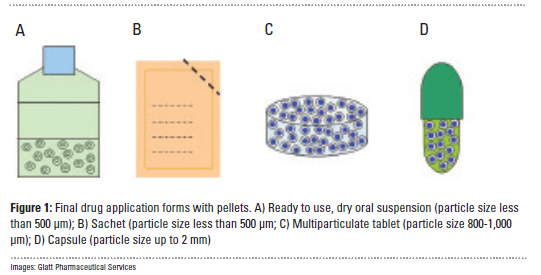
In contrast to classic single-unit dosage forms such as tablets, the dosage of the drug substance in multi-particulate systems is divided on a plurality of subunits – typically consisting of thousands of spherical pellet particles with a diameter of between 100 and 2,000 μm. This means that non-disintegrating, monolithic single-unit forms retain their structure in the digestive tract, whereas the multi-particular preparations consist of numerous sub-units which disperse after administration....
26. June 2018
More and more pharmaceutical products are reaching the market as multiparticulate dosage forms, mainly as pellets. The healthcare sector also frequently selects pellets as the optimal marketable form of functional food. As there are quite a number of existing techniques relating to the production of pellets, it is often very difficult for a formulator or marketing manager to make a choice, since every technique claims to be optimal.
15. March 2018
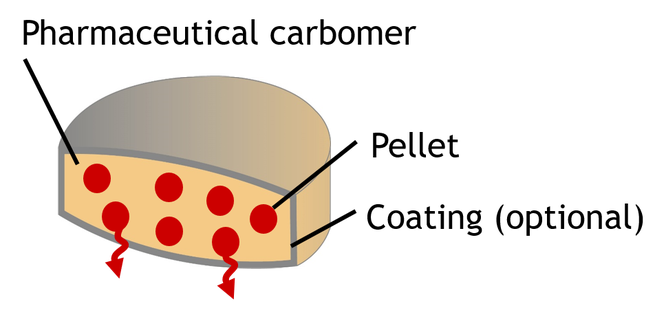
The Orogenta® Technology enables to achieve zero-order release MUPS tablets coating of the embedded multiparticulates.
04. March 2018
With manufacturers beginning to improve their understanding of the uses of multiparticulate products, alongside more research and progressive advancements being made, the future looks bright for pelletisation as a technique in drug development. Here, Dr Beata Vladovicova talks about the wide-ranging advantages of pelletisation, whilst also exploring the use of extrusion-spheronisation to produce these types of drugs. She also discusses the challenges faced in the development of pellets and why s
25. October 2017
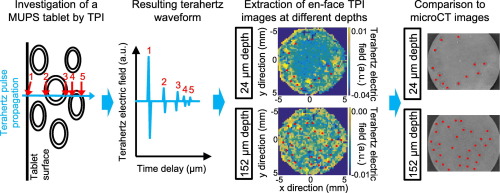
Terahertz pulsed imaging (TPI) was applied to analyse the inner structure of multiple unit pellet system (MUPS) tablets. MUPS tablets containing different amounts of theophylline pellets coated with Eudragit® NE 30 D and with microcrystalline cellulose (MCC) as cushioning agent were analysed
11. September 2017
The purpose of this work was to develop multi unit particulate formulation using fluid bed processor for the controlled release of Metoprolol succinate and to understand the impact of formulation parameters on the critical quality attributes using a quality-by-design approach.
05. July 2017
Acid-labile drugs are easily degraded in acidic medium, which have been mainly formulated as enteric-coated dosage forms for oral administration. The objective of this study was to prepare oral multiparticulate formulations of acid-labile drugs, using ilaprazole, lansoprazole and rabeprazole sodium as model drugs.
27. October 2016
Abstract Multiple unit pellet system(s), MUPS, is one of the most commonly used means for achieving modified drug release through oral administration. MUPS is usually delivered as capsules that contain drug bearing beads coated with a release modulating functional layer. While the routine manufacture of tablets of MUPS by direct compression is highly desired, it is faced with the problem of poor tableting properties or altered drug release behavior due to the rupture of the functional coating...
04. October 2016
Abstract In the present study the applicability of multispectral UV imaging in combination with multivariate image analysis for surface evaluation of MUPS tablets was investigated with respect to the differentiation of the API pellets from the excipients matrix, estimation of the drug content as well as pellet distribution, and influence of the coating material and tablet thickness on the predictive model. Different formulations consisting of coated drug pellets with two coating polymers...