- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. July 2018
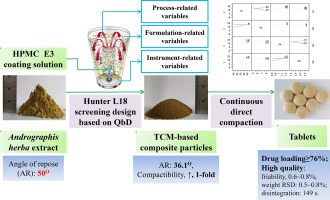
The Andrographis herba extract (AHE), a traditional Chinese medicine (TCM), was developed to directly compactible powders by fluid bed coating with 6% to 12% hydroxypropyl methylcellulose (HPMC). The process-, instrument-, and formulation-related variables of the coating process were simultaneously optimized with the Hunter L18 screening design. Yield (Y1), compactibility (Y2), and angle of repose (Y3) were measured as the responses. The optimized variables were 50 °C for inlet air...
19. February 2018
The objective of this study was to assess the efficacy and the capability of a novel ethylcellulose-based dry-coating system to obtain prolonged and stable release profiles of caffeine-loaded pellets. Lauric and oleic acids at a suitable proportion were used to plasticize ethylcellulose. The effect of coating level, percentage of drug loading, inert core particle size, and composition of the coating formulation including the anti-sticking agent on the drug release profile were fully...
02. September 2017
Abstract Capping is a common problem in the manufacture of some types of tablets and unless resolved, the tableting process cannot proceed. Hence, all factors that can help to lessen the likelihood of capping without unnecessarily reduce turret speed and/or compaction force would be tenable. This study investigated the influence of tablet punch configuration on mitigation of tablet capping. Tablets were prepared from high-dose paracetamol-potato starch granules in a rotary tablet press with...
29. April 2017
Abstract Thoughtfully designed early clinical formulations not only meet the needs of the study at hand and inform the development of the commercial product, but can influence the direction of the clinical program as well as provide further guidance to potential backups still in exploratory stages. This chapter focuses on the various types of early clinical formulations, why they are developed, and how the preclinical formulation space helps to guide initial clinical formulation selection....
05. April 2017
Abstract We investigated the effectiveness of using Carr’s flowability index (FI) and practical angle of internal friction (Φ) as indexes for setting the target Mg-St mixing time needed for preparing tablets with the target physical properties. We used FI as a measure of flowability under non-loaded conditions, and Φ as a measure of flowability under loaded conditions for pharmaceutical powders undergoing direct compression with varying concentrations of Mg-St and mixing times. We evaluated...
20. March 2017
Abstract Purpose A near-infrared (NIR) spectroscopic method was developed for real time analysis of the active pharmaceutical ingredient (API) in blends from a continuous manufacturing process. The sampling and analytical errors of these determinations were estimated through variographic analysis. Methods Thirty-three calibration blends were prepared in laboratory scale equipment with a concentration range spanning from 70 to 130% of API target concentration. The NIR calibration model was...
14. March 2017
Abstract Using pharmaceutical salts in solid dosage forms can raise stability concerns, especially salt dissociation which can adversely affect the product performance. Therefore, a thorough understanding of the salt instability encountered in solid state formulations is imperative to ensure the product quality. The present article uses the fundamental theory of acid base, ionic equilibrium, relationship of pH and solubility as a starting point to illustrate and interpret the salt formation and...
08. March 2017
ABSTRACT: Topiramate (TPM) is effective for multiple seizure types and epilepsy syndromes in children and adults. Topiramate has adverse effects (including cognitive, depression, renal stones), but many of these are low incidence when started at a low dose and slowly titrated to 100 to 200 mg/day. Also, TPM has proven benefit for migraine, obesity, eating disorders, and alcohol use disorders, which can be comorbid in patients with epilepsy and may also be effective in subpopulations within...
20. February 2017
The main objective of the present study was to apply QbD methodology in the development of once-a-day sustained release quetiapine tablets. The quality target product profile (QTPP) was defined after the pharmaceutical properties and kinetic release of the innovator product, Seroquel XR 200 mg. For the D-optimal experimental design, the level and ratio of matrix forming agents and the type of extra granular diluent were chosen as independent inputs, which represented critical formulation...
03. February 2017
ABSTRACT Co-processing is one of the ways to develop new excipients for the pharmaceutical industry. Co-processed excipients are a combination of established two or more excipients through physical mixing or co-process technology. Co-processed excipients has no change in chemical structure, it only changes physical properties of the final product. Co-processing technology could lead to formation of co-processed excipient with superior physical properties compared to simple physical mixtures of...