- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. July 2018
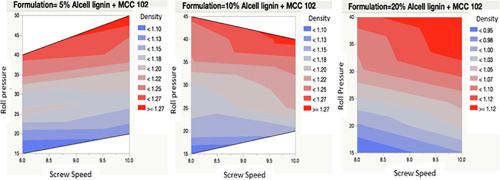
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
05. January 2017
ABSTRACT Quality of pharmaceutical product is very important because pharmaceuticals drugs should be safe and therapeutically active formulation performance should be consistent and predictable. Final product quality depends on all ingredients which is used for making the final product tablet. Final product tablet is made by the addition of bulk drug and excipients. The continuous evolution of the bulk drugs and excipients can only ensure the quality of final product. Moisture content of...
14. December 2016
Abstract The purpose of the study was to develop and evaluate a tripartite novel excipient for direct compression of salbutamol tablets. Various batches (A-E) of the novel excipient was prepared by co-processing varying concentrations of okro gum with gelatinized maize starch and lactose using co-precipitation method. The novel excipient powders were subjected to some physicochemical evaluations. Batches (F-H) of the physical mixture of the novel excipient ingredients were also prepared. The...
04. August 2016
Objectives: To develop orodispersible films (ODF) based on hydrophobic polymers with higher stability to ordinary environmental humidity conditions without compromising their fast disintegration time. Methods: A quality by design approach was applied to screen three different formulations each one based on a different hydrophobic polymer: polyvinyl acetate, methacrylate-based copolymer and shellac. The screening formulations were characterized regarding their mechanical properties, residual...
04. July 2016
Context: Orodispersible films have gained increasing relevance as a novel dosage form. Associated to their particular processing and multicomponent composition, there are a vast number of reasons to establish helpful development guidance, gathering simultaneously the recent pharmaceutical regulatory trends. Objective: This study aimed characterize marketed orodispersible films in order to provide essential information about a clear definition of product critical quality attributes (CQAs)....
29. May 2016
Abstract: Preparation of drug nanoparticles via wet media milling (nanomilling) is a very versatile drug delivery platform and is suitable for oral, injectable, inhalable, and buccal applications. Wet media milling followed by various drying processes has become a well-established and proven formulation approach especially for bioavailability enhancement of poorly water-soluble drugs. It has several advantages such as organic solvent-free processing, tunable and relatively high drug loading,...