- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
19. September 2018
As a widely used excipient in pediatric formulations, propylene glycol functions as a solvent, emulsifier, humectant, and hygroscopic agent. It is a clear, colorless liquid whose properties enable it to have pharmacodynamic applications. Oftentimes, propylene glycol is combined with other mediations to enhance its penetration. For instance, a combination of 20% propylene glycol and 5% lactic acid in a semiocculusive cream base is used as a highly effective and well-tolerated keratolytic in...
06. September 2018
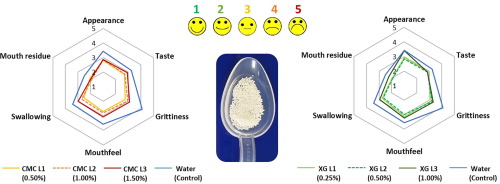
Multiparticulate formulations based on pellets, granules or beads, could be advantageous for paediatrics, geriatrics and patients with swallowing difficulties. However, these formulations may require suitable administration media to facilitate administration. The aim of this work was to investigate the effect of administration media properties on palatability and ease of swallowing of multiparticulates. A range of vehicles were developed using xanthan gum (XG) and carboxymethyl cellulose (CMC)...
15. August 2018
To attain effective and safe pharmacotherapy, formulations in (pre)term neonates should enable extensive dose flexibility. During product development and subsequent authorization and clinical use of such formulations, there is also a need for informed decisions on excipient exposure: in addition to the need to improve the knowledge on active compounds, there is a similar need to improve the knowledge on excipients in neonates. Excipients are added to formulations as co-solvent, surfactant,...
26. June 2018
More and more pharmaceutical products are reaching the market as multiparticulate dosage forms, mainly as pellets. The healthcare sector also frequently selects pellets as the optimal marketable form of functional food. As there are quite a number of existing techniques relating to the production of pellets, it is often very difficult for a formulator or marketing manager to make a choice, since every technique claims to be optimal.
02. March 2018
For orally administered drugs, the rate and extent of absorption are governed by the physiology of the gastrointestinal tract, the characteristics of the dosage form and the physico-chemical properties of the drug. This thesis primarily aimed to improve the mechanistic understanding and the predictability of processes involved in the absorption of orally administered drugs using a population modeling approach. A secondary aim was to propose an optimized dosing regimen for first line anti-tubercu
02. October 2017
Experienced CDMO partner for solid dosage forms - Innovative solutions for multiparticulates, pellets and micro pellets
Pediatric formulation - Quality by Design and Scale up concepts - Clinical trial and commercial bulk manufacturing - Controlled substances / organic solvents handling- GMP / FDA sites in Binzen/Germany and Ramsey/USA
22. February 2017
Abstract An innovative pediatric oral formulation of hydrochlorothiazide (HCT) (2 mg/mL), endowed with improved bioavailability and sustained release properties and suitable for the hypertension treatment in pediatric patients, was developed by combining the drug-cyclodextrin complexation and the incorporation of the complex into Solid Lipid Nanoparticles (SLN). Precirol®ATO5-based SLN, with two different surfactants (Pluronic®F68 and Tween®80) loaded with the drug as such or as binary...
20. July 2016
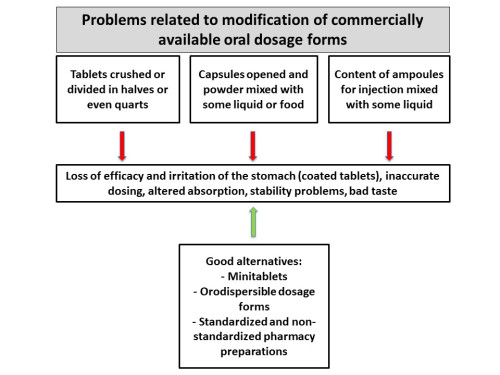
For many drugs commonly used in pediatrics, a suitable drug formulation or dosage strength is not commercially available. For that reason, children frequently receive medicines that are designed for adults, a practice called “unlicensed” or “off-label use.” The dose of commercially available products is adapted, mostly based on the child’s bodyweight, thereby neglecting differences in pharmacokinetic and pharmacodynamic parameters. Nowadays, serious attempts are being made to develop...
13. March 2016
Background Neonates may respond differently from adults to drug components. Hence, ingredients that seem safe in adults may not be safe in this age group. Objective To describe the content of harmful excipients in drugs used in our neonatal wards and compare the daily dose a neonate may receive with the accepted daily intake (ADI) in adults. Methods All drugs included in the hospital’s neonatal treatment guide were reviewed, using information from the package inserts or the summary of product...
10. February 2016
The STEP stands for Safety and Toxicity of Excipients for Paediatrics. The STEP database is a user-designed free resource that compiles the safety and toxicity information of excipients that is manually extracted from selected information sources. Three tier quality control procedures are implemented from information retrieval level to entry of the specific data in the database. More