- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
28. May 2018
Printing technologies have recently emerged in the development of novel drug delivery systems toward personalized medicine, to improve the performance of formulations, existing bioavailability patterns, and patients’ compliance. In the context of two-dimensional printing, this article presents the development of buccal films that are designed to efficiently deliver a class II compound (diclofenac sodium), according to the Biopharmaceutics Classification System (BCS), to the oral cavity. The...
04. April 2018
During the development of parenteral dosage forms, different physicochemical studies are required to ensure stable, effective and safe formulations. The osmolality of this kind of dosage form should bear a close similarity to the body fluids to prevent local irritation, pain or even more significant side effects like endothelial damage. The osmotic studies performed in Polyethylene glycol 400 (PEG 400), Polyethylene glycol 4000 (PEG 4000), Poloxamer 407 (P407), Sodium Hyaluronate (SH),...
16. March 2018
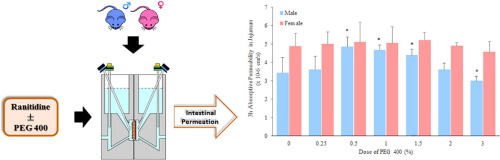
Sex-related differences in drug pharmacokinetics, especially regarding absorption and metabolism, are in part caused by a differential expression and/or activity of membrane transporters between males and females (Soldin and Mattison, 2009, Morris et al., 2013). Certain transporters are known to be influenced by formerly considered “inert” pharmaceutical excipients with which drugs are co-formulated.
20. December 2017
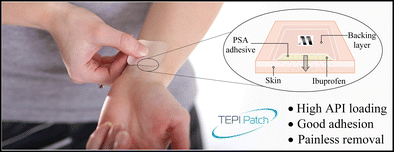
The main objective of this present study was the investigation of potential novel transdermal patch technology (TEPI®) delivering ibuprofen as the active pharmaceutical ingredient (API) using a novel poly(ether-urethane)-silicone crosslinked pressure-sensitive adhesive (PSA) as the drug reservoir in a solvent-free manufacturing process.
19. July 2017
The pharmaceutical excipient, polyethylene glycol 400 (PEG 400), unexpectedly alters the bioavailability of the BCS class III drug ranitidine in a sex-dependent manner