- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. July 2018
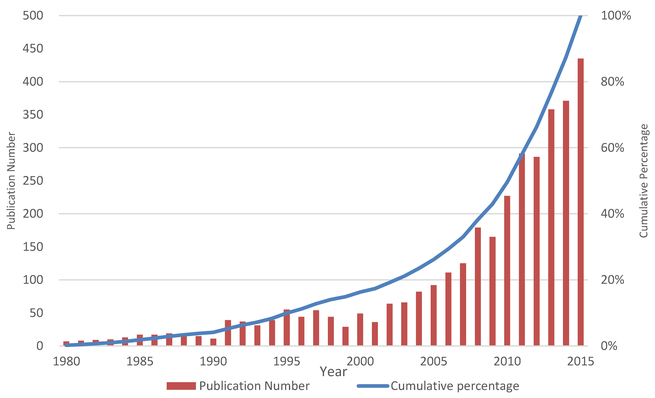
Solid dispersions are an effective formulation technique to improve the solubility, dissolution rate, and bioavailability of water-insoluble drugs for oral delivery. In the last 15 years, increased attention was focused on this technology. There were 23 marketed drugs prepared by solid dispersion techniques. Objective: This study aimed to report the big picture of solid dispersion research from 1980 to 2015. Method: Scientific knowledge mapping tools were used for the qualitative and the...
16. February 2018
Here you find some abstracts of Engineering of "Biomaterials for Drug Delivery Systems - beyond Polyethylene Glycol". 1 – PEGylated “stealth” nanoparticles and liposomes Through nanomedicine, game-changing methods are emerging to deliver drug molecules directly to diseased tissue. The targeted delivery of drugs and imaging agents via drug carrier-based platforms is among the most promising of these methods. Such drug delivery systems can now be synthesized from a wide range of different...
21. January 2018
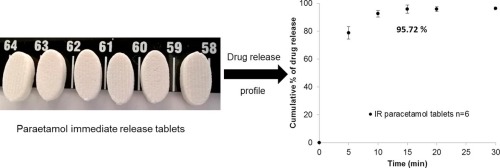
The manufacture of immediate release high drug loading paracetamol oral tablets was achieved using an extrusion based 3D printer from a premixed water based paste formulation. The 3D printed tablets demonstrate that a very high drug (paracetamol) loading formulation (80% w/w) can be printed as an acceptable tablet using a method suitable for personalisation and distributed manufacture.
11. December 2017
Nowadays most of the drug substances are coming into the innovation pipeline with poor water solubility. Here, the influence of excipients will play a significant role to improve the dissolution of poorly aqueous soluble compounds. The drug substance needs to be dissolved in gastric fluids to get the better absorption and bioavailability of an orally administered drug. Dissolution is the rate-controlling stage for drugs which controls the rate and degree of absorption.
02. May 2017
Highlights
• Binder properties were correlated to granule and tablet properties.
• Groups of binders behave divergent regarding granule and tablet properties.
• Povidone based polymers seem to have another binding behaviour.
• Disintegration of tablets was explained by particle size and viscosity effects.
• Changes in particle size caused larger effects for small-sized binders on tablets.
20. March 2017
ABSTRACT The study carried out here was focused on developing conventional monolithic controlled release matrix tablet of Atorvastatin calcium using carbomer as release controlling polymer. This system ensures the drug release at the alkaline pH region where the drug has got maximum solubility. Further the study was concentrated on comparing the impact of gelling agent polyvinyl pyrrolidone on drug release. Quality by design tools were considered during formulation development and the polymer...
21. July 2016
20.07.2016 Rosenberg, Germany - July 20, 2016 - JRS PHARMA, headquartered in Germany, has entered into a joint venture agreement with SSP, headquartered in Chongqing, China, for the commercialization of povidone products. The new JV company is Star-Tech & JRS Specialty Products. Equipped with innovative technology and a state-of-the-art GMP-compliant production facility, Star-Tech & JRS Specialty Products specializes in the manufacture of high quality povidones with CEP certification...
17. February 2016
This study evaluated the effects of changing the amount of added water for moisture activated dry granulation (MADG) on the resulting granule characteristics and tablet properties. Water soluble fillers and binders were pre-mixed in a granulator, and the binder was activated by a small amount of water to form granules. The amount of added water was defined as the percentage of granulation water by mass to charged powder. Granules made with 0.0%, 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, and 5.0% (w/w)...
30. November 2015
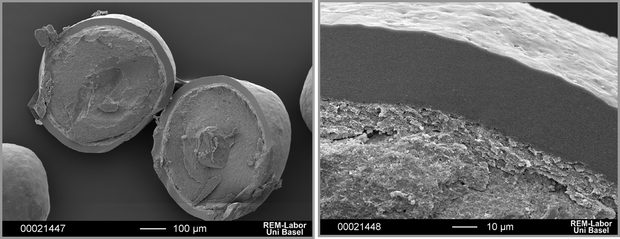
Pharmaceutical solid dosage forms are commonly coated to modify the release of drugs. Due to the disadvantages of coated single-unit dosage forms, such as occurrences of dose dumping and local irritation, coated multi-particulates are preferred. Coated multi-particulates can eventually be filled into capsules or compressed into tablets. The latter is more desirable as unit production costs of tablets are considerably lower and machinery more easily available. More