- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
19. August 2018
The staff of the FDA’s Center for Drug Evaluation and Research (CDER) always tries to utilize cutting-edge science and up-to-date process management, befitting our stature as the global “gold standard” in drug regulation. Maintaining that standard requires us to keep up with evolving technology and the latest scientific, medical and regulatory advances. Current factors impacting drug development include the genomic revolution, the rise of targeted therapy, the availability of digital...
08. August 2018
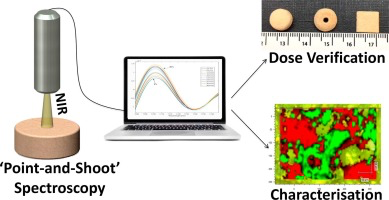
Three-dimensional printing (3DP) has the potential to cause a paradigm shift in the manufacture of pharmaceuticals, enabling personalised medicines to be produced on-demand. To facilitate integration into healthcare, non-destructive characterisation techniques are required to ensure final product quality. Here, the use of process analytical technologies (PAT), including near infrared spectroscopy(NIR) and Raman confocal microscopy, were evaluated on paracetamol-loaded 3D printed cylindrical...
17. July 2018
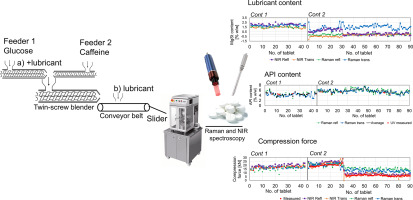
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
07. July 2018
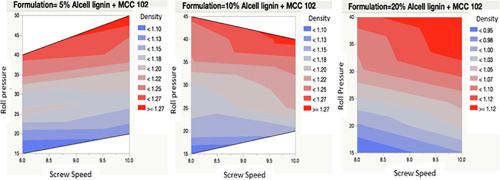
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
16. June 2018
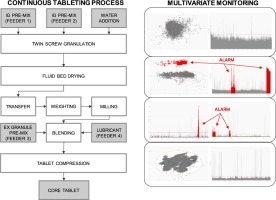
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of...
30. May 2018
The aim of this study was to develop a palatable donepezil (DP) orodispersible film (ODF) to facilitate the swallowing process and investigate the effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Complexation of DP with hydroxypropyl-β-cyclodextrin (HP-β-CD) was applied to mask the bitter taste then the prepared complexes were incorporated into ODF using solvent casting method. The taste-masking efficiency was evaluated by e-tongue; meanwhile the...
05. March 2018
This study presents a framework for process and product development on a continuous direct compression manufacturing platform. A challenging sustained release formulation with high content of a poorly flowing low density drug was selected. Two HPMC grades were evaluated as matrix former: standard Methocel CR and directly compressible Methocel DC2. The feeding behavior of each formulation component was investigated by deriving feed factor profiles.
01. March 2018
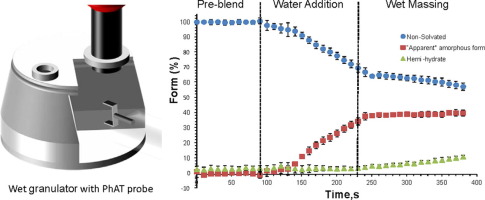
Form changes during drug product processing can be a risk to the final product quality in terms of chemical stability and bioavailability. In this study, online Raman spectroscopy was used to monitor the form changes in real time during high shear wet granulation of Compound A, a highly soluble drug present at a high drug load in an extended release formulation. The effect of water content, temperature, wet massing time and drying technique on the degree of drug transformation were examined.
18. November 2017
by Innopharmatechnology & Glatt @ PMEC India 2017 Learn about the latest Process Analytical Technology (PAT) technical and application developments for in-line PSD and Moisture measurement in fluid bed batch and continuous powder processes. More
07. November 2017
Over the last few decades, hot melt extrusion (HME) has emerged as a successful technology for a broad spectrum of applications in the pharmaceutical industry. As indicated by multiple publications and patents, HME is mainly used for the enhancement of solubility and bioavailability of poorly soluble drugs. This review is focused on the recent reports on the solubility enhancement via HME and provides an update for the manufacturing/scaling up aspects of melt extrusion. In addition, drug...