- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
19. August 2018
The staff of the FDA’s Center for Drug Evaluation and Research (CDER) always tries to utilize cutting-edge science and up-to-date process management, befitting our stature as the global “gold standard” in drug regulation. Maintaining that standard requires us to keep up with evolving technology and the latest scientific, medical and regulatory advances. Current factors impacting drug development include the genomic revolution, the rise of targeted therapy, the availability of digital...
30. July 2018
Injection site reactions (ISRs) and other adverse side-effects are commonly observed during therapy with biologics. These hypersensitivity related side-effects can vary from simple rash to life-threatening anaphylactic reaction, and may be linked to the immunogenicity of the drug including formation of antidrug antibodies. Reactions can also occur as a consequence of excipients in the product. We report the case of a patient who developed erythematous ISRs to both commercial PCSK9i formulations...
20. March 2017
Abstract Local ocular delivery of cyclosporine A (CsA) is the preferred method for CsA delivery as a treatment for ocular inflammatory diseases such as uveitis, corneal healing, vernal keratoconjunctivitis and dry eye disease. However, due to the large molecular weight and hydrophobic nature of CsA and the natural protective mechanisms of the eye, achieving therapeutic levels of CsA in ocular tissues can be difficult. This review gives a comprehensive overview of the current products available...
24. October 2016
Abstract The rapidly growing medical expense going with aging population in Japan requires the use of generic products derived from off-patent active pharmaceutical ingredients (APIs) formulations to reduce the financial burden for the national health insurance system, while at the same time avoiding undermining the quality of medical care. This article provides an overview of the regulatory approaches available to confirm the quality of generic products and gain their greater acceptance by...
22. August 2016
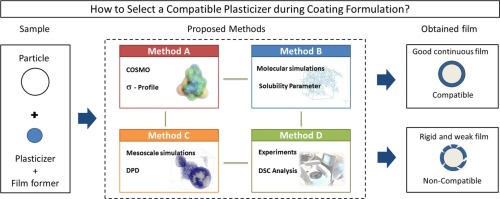
Abstract During the formulation of solid dosage forms coating, plasticizers are added to the film forming polymer to improve the mechanical properties of the coating shell of the drug product. For the coating formulation to be successful and in order to produce flexible continuous film, the plasticizer should be compatible with the film forming polymer (i.e. high plasticizer-polymer miscibility in solid dispersion) (McGinity and Felton, 2008). This paper proposes and compares different...
29. May 2016
Abstract Excipients represent diverse classes of molecules, small molecules or macromolecules, with versatile structures within a given class and are from natural, semi-synthetic and synthetic sources. They are essential ingredients in drug products independently of the route of administration where both traditional and non-traditional uses are present. Beyond their traditional use as formulation and manufacturing aids, certain excipients exhibit biological effects and thus can be used either...
27. March 2016
In “The Lives of a Cell”, L. Thomas restated a hitherto prosaic observation as a profound conjecture, i.e, that mitochondria - the earliest aerobic bacteria - may have “created” us as a ‘symbiont utilitarian layer’ for their survival. The same can be argued for the bacteria resident in the gastrointestinal system which, to put it bluntly, may have ‘created’ us in order to feed them. There are more bacteria in the human body (>500 species) than there are human cells. Most of...
03. February 2016
The chairs of each of the 8 Special Interest Groups of the Board of Pharmaceutical Sciences of the International Pharmaceutical Federation have compiled opinions with regard to major challenges for the pharmaceutical sciences over the next 5-10 years. Areas covered are drug design and discovery, natural products, formulation design and pharmaceutical technology, pharmacokinetics /pharmacodynamics and systems pharmacology, translational and personalized medicine, biotechnology, analytical...
03. February 2016
Amorphous products and particularly amorphous solid dispersions are currently one of the most exciting areas in the pharmaceutical field. This approach presents huge potential and advantageous features concerning the overall improvement of drug bioavailability. More
05. January 2016
In coating and agglomeration processes, the properties of the final product, such as solubility, size distribution, permeability and mechanical resistance, depend on the process parameters and the binder (or coating) solution properties. These properties include the type of solvent used, the binder composition and the affinity between its constituents. More