- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. August 2018
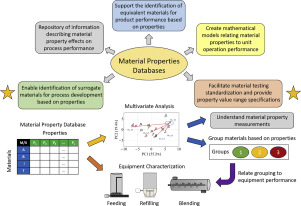
Material properties are known to have a significant impact on pharmaceutical manufacturing performance, particularly for solid product processes. Evaluating the performance of a specific material, for example an active pharmaceutical ingredient or excipient, is critical during development stages in order to determine the impact of material properties on the process. However, materials may be scarce during the early stages of process development due to high cost, unavailability, import...
05. April 2017
Abstract We investigated the effectiveness of using Carr’s flowability index (FI) and practical angle of internal friction (Φ) as indexes for setting the target Mg-St mixing time needed for preparing tablets with the target physical properties. We used FI as a measure of flowability under non-loaded conditions, and Φ as a measure of flowability under loaded conditions for pharmaceutical powders undergoing direct compression with varying concentrations of Mg-St and mixing times. We evaluated...
03. April 2017
Abstract Capping or lamination is an unsolved common problem in tablet manufacturing. Knowledge gaps remain despite an enormous amount of effort made in the past to better understand the tablet capping/lamination phenomenon. Using acetaminophen – containing formulations, we examined the potential use of a compaction simulator as a material-sparing tool to predict capping occurrence under commercial tableting conditions. Systematical analyses of the in-die compaction data led to insight on the...
01. October 2016
Abstract Flashing at tablet edges, an inevitable phenomenon when tableting a plastically deforming material under a high pressure, can introduce significant errors to tablet density and porosity determinations due to overestimated tablet thickness when tablet were measured out-of-die. Errors in tablet density determination also lead to errors in true density obtained by the Sun method, which is suitable for water-containing solids. Errors in true density and tablet porosity propagate to...
19. May 2016
In the present investigation, liquisolid compact technique is investigated as a tool for enhancement of dissolution of poorly water-soluble drug, Deflazacort. Deflazacort liquisolid tablets were prepared using propylene glycol as non-volatile liquid vehicle, microcrystalline cellulose (Avicel PH 102) as carrier material, colloidal silicon dioxide (Aerosil 200) as coating material and sodium starch glycolate as super disintegrant. The prepared liquisolid compacts were evaluated for bulk density,...
21. March 2016
Abstract. The crystal structures of active pharmaceutical ingredients and excipients should be strictly controlled because they influence pharmaceutical properties of products which cause the change in the quality or the bioavailability of the products. In this study, we investigated the effects of microcrystalline cellulose (MCC) crystallinity on the hydrophilic properties of tablets and the hydrolysis of active pharmaceutical ingredient, acetylsalicylic acid (ASA), inside tablets by using...