- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
25. June 2018
Excipients are an inactive substances formulated along with the active pharmaceutical ingredient(s) (APIs) of medication for bulking up of formulations or to give a therapeutic enhancement of API in the final dosage form. Almost of all the marketed products contain excipients thereby the excipients play a crucial role in the manufacture of medications by helping to preserve the efficacy, safety and functionality of the APIs. Excipients categorized as compendial excipients (official in...
06. September 2015
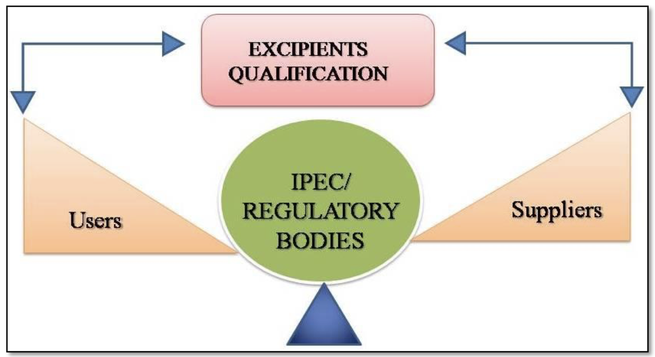
Pharmaceutical excipients have different functions within a drug formulation, consequently they can influence the manufacturability and/or performance of medicinal products. Therefore, critical to quality attributes should be kept constant. Sometimes it may be necessary to qualify a second supplier, but its product will not be completely equal to the first supplier product. More
09. June 2015
Highlights • Qualification of entire processes based on CQAs of the final product (excipient). • Use of analytical methods (Raman, X-ray- and laser diffraction) with MVDA. • The value of such combined strategy is to produce a better diagnostics of quality. • Evidence-driven diagnostics of which process delivers a more consistent end-product. • Strategy for a risk-based supplier qualification using an analytical data matrix. Read more