- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. August 2018
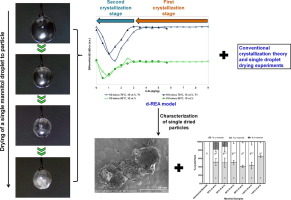
In this paper, we explain the crystallization process of mannitol during convective droplet drying based on the crystallization kinetics calculated from two mathematical models coupled with experimental investigations. A novel differential-reaction engineering approach was developed, correlating the mannitol crystallization behavior to the deviation of droplet drying kinetics at different drying temperatures. The model was compared with a conventional glass transition-based model and the...
01. October 2017
In general, it is an important criterion that excipients remain inert throughout the shelf life of the formulated pharmaceutical product. However, depending on the functionality in chemical structure of active drug and excipients, they may undergo interaction. The well-known Maillard reaction occurs between a primary amine with lactose at high temperature to produce brown pigments. The reactivity of Maillard reaction may vary depending on the concentration as well as other conditions....
22. March 2016
A number of intravenous immunoglobulin preparations are stabilized with sugar additives that may lead over time to undesirable glycation reactions especially in liquid formulation. This study aimed to evaluate the reactivity of sugar excipients on such preparations in condition of temperature, formulation and concentration commonly used for pharmaceutical products. Through an innovative LC-MS method reported to characterize post-translational modifications of IgGs Fc/2 fragments, a stability...
03. December 2015
Background Many excipients have been reported to induce drug hypersensitivity (e.g. colouring additives, preservatives). Colloidal silica has never been reported to induce drug hypersensitivity reactions. Case report We report herein a 40-year-old patient who developed a skin eruption 2 days after Voltarene® (diclofenac) intake, confirmed by a positive patch test. Investigation of cross reactivity, assessed by patch testing to other non steroidal anti-inflammatory drugs, have showed a positive...
08. July 2015
Formulations of the same active pharmaceutical ingredients (API) may contain a variety of inactive pharmaceutical ingredients (IPI) or excipients. Package inserts are important sources of information to clinicians and should provide details of both the API and the excipients in the pharmaceutical preparation. The Medicine Control Council's published guidelines recommend the inclusion of excipients (qualitative) in the package insert, but this is not adequately enforced. Excipient-related...
24. April 2015
Formulations of the same active pharmaceutical ingredients (API) may contain a variety of inactive pharmaceutical ingredients (IPI) or excipients. Package inserts are important sources of information to clinicians and should provide details of both the API and the excipients in the pharmaceutical preparation... http://reference.sabinet.co.za/sa_epublication_article/caci_v28_n1_a7