- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. July 2018
Soluplus® has been approved in generics in Taiwan and Argentina and now filed in Brazil. Would like to be kept updated on Soluplus® regulatory status or need further Information? Sign up here!
19. July 2018
Did you know that Soluplus® is already approved in generics in Taiwan and Argentina and now filed in Brazil? Check the submentioned Soluplus® one pager with updated regulatory status per country. Soluplus® Key Customer Benefits Outstanding solubilization properties, especially for poorly soluble APIs Enables bioavailability enhancement Ideal for hot melt extrusion and all standard granulation techniques Market proven solution for unique formulation challenges Would like to be kept updated on...
30. May 2018
18-19 September 2018 in Cologne - Germany OBJECTIVES Implementation of GMP in an excipient manufacturing site Qualification and auditing of suppliers Analytical data and certificates of analysis for excipients The European Pharmacopoeia’s General Methods Modernisation Programme USP – Strategies and Opportunities for Excipient Standard Setting Particulate matter in pharmaceutical starting materials and drug products Regulatory and technical challenges for excipients in parenteral...
26. May 2018
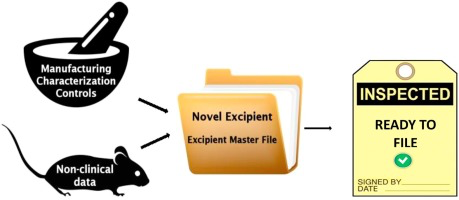
Novel excipients are indispensable in development of modern, advanced drug delivery systems and biotechnology-derived drugs. Although numerous novel excipients are developed for pharmaceutical use, they are not frequently seen in medicinal products due to the strict regulatory requirements and perception that their use makes new product evaluation more complex with risk of delays in the approval process. Regulators regard novel excipients as new substances and whenever new excipient is used in...
25. April 2018
To support the practical implementation of the ICH Q3D guideline, which describes a risk-based approach to the control of elemental impurities in drug products, a consortium of pharmaceutical companies has established a database to collate the results of analytical studies of the levels of elemental impurities within pharmaceutical excipients. This database currently includes the results of 26723 elemental determinations for 201 excipients and represents the largest known, and still rapidly...
18. March 2018
This presentation will explain the definition of pharmaceutical excipients. Also, it shows the main regulation institutions and existing regulation. It shows the main roles of pharma excipients as well as the ideal properties of pharma excipients. This first part ends with a short view of the excipient market.
26. September 2017
In general, it is an important criterion that excipients remain inert throughout the shelf life of the formulated pharmaceutical product. However, depending on the functionality in chemical structure of active drug and excipients, they may undergo interaction.
22. November 2016
Currently, because globalization, the pharmaceutical industry is facing enormous challenges to comply with regulatory matters. Reduced patent life and overall decreased profitability of newly discovered drugs are also forcing the pharmaceutical industry to shorten the drug development time with maximum throughput. Therefore, continuous manufacturing (CM) processes via hot melt extrusion (HME) can be a promising alternative for achieving these goals. HME offers solvent-free green technology with...
22. September 2016
Abstract: Background: In this era of evolution, quality and safety of pharmaceutical dosage forms have been given a prime importance. In this context, a detailed knowledge about physical and chemical properties of excipients is not sufficient, but information about safety and regulatory status of these materials is now essential to know. The present work will be beneficial to focus more on the safety, quality and stability of pharmaceutical product. Objective: Study of regulatory mechanism for...
27. June 2016
Abstract Pharmaceutical excipients contribute unique functionalities to formulations, thereby largely determining the drug products quality and influencing its safety and efficacy. Changes and variations of excipients in licensed products are, therefore, placed under strict regulatory control. This article presents a proposed regulatory mechanism for pharmaceutical excipients by United States of Food and Drug Administration (USFDA). In Unites States, excipients are regulated by United States of...