- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. January 2018
Excipients serve a critical role in the production of final dosage forms for drug products and biologics. They facilitate the manufacturing process (e.g., anticaking agents) and protect, support, and enhance stability. They may also improve bioavailability. In addition, excipients help maintain the safety, or function, of the product during storage and use.
No longer characterized as inert accompaniments to an active pharmaceutical ingredient (API), excipients are the target of an intensified p
09. November 2017
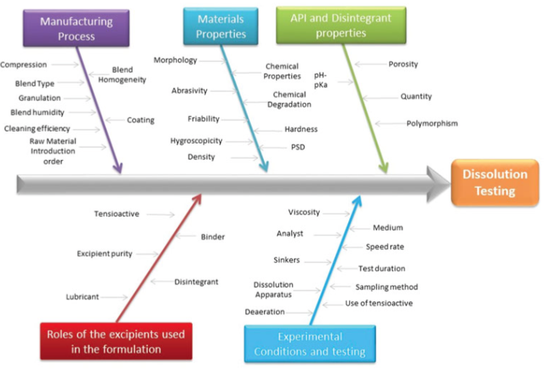
This study applied the concept of Quality by Design (QbD) to tablet dissolution. Its goal was to propose a quality control strategy to model dissolution testing of solid oral dose products according to International Conference on Harmonization guidelines. The methodology involved the following three steps.
16. October 2017
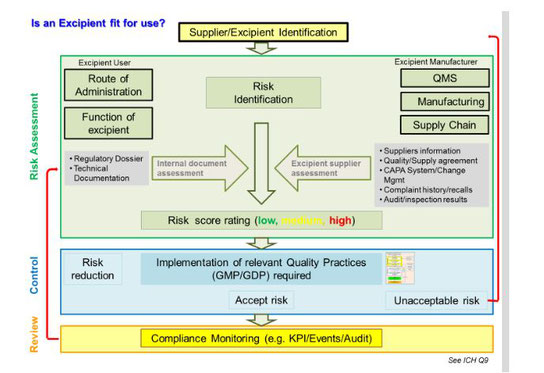
Risk assessment is already a hot topic for excipients and becoming more important all the time. Excipient makers have not always had the same level of need or opportunity as excipient users (pharmaceutical companies) to conduct risk assessments within their manufacturing processes.
21. August 2017
Excipients / inactive ingredients are an integral part of practically all medicines. Although being a functional part of a formulation, excipients have been regarded as pharmacologically and chemically indifferent within the formulation.