- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. January 2018
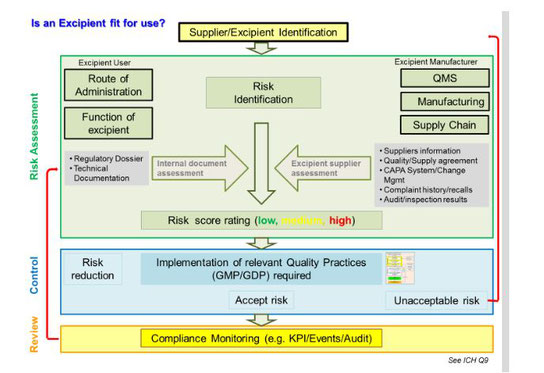
Excipients serve a critical role in the production of final dosage forms for drug products and biologics. They facilitate the manufacturing process (e.g., anticaking agents) and protect, support, and enhance stability. They may also improve bioavailability. In addition, excipients help maintain the safety, or function, of the product during storage and use.
No longer characterized as inert accompaniments to an active pharmaceutical ingredient (API), excipients are the target of an intensified p
09. November 2017
One of the most promising strategies to improve the bioavailability of active pharma- ceutical ingredients is based on the association of the drug with colloidal carriers, for example, polymeric nanoparticles, which are stable in biological environment, protec- tive for encapsulated substances and able to modulate physicochemical characteristics, drug release and biological behaviour.