- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
05. September 2018
The predictability of preformulation screening tools for polymer selection in amorphous solid dispersions (ASD) regarding supersaturation and precipitation was systematically examined. The API-polymer combinations were scaled up by means of hot-melt extrusion and spray-drying to verify the predictions. As there were discrepancies between a solvent-based screening and performance of ASD, a new screening tool with improved predictability at minimal investments of time and material is presented....
25. July 2018
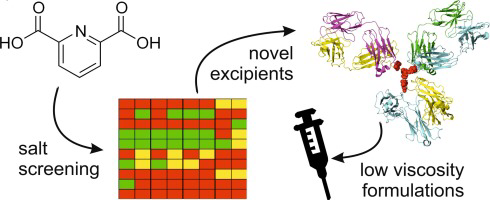
Concentrated monoclonal antibody (mAb) solutions can lead to high viscosity as a result of protein-protein interactions and pose challenges for manufacture. Dipicolinic acid (DPA, pyridine-2,6-dicarboxylic acid) is a potential excipient for reduction of protein solution viscosity and here we describe new DPA salts with improved aqueous solubility. Crystallinity and solubility screens identified ethanolamine and diethanolamine as two promising counterions which generated crystalline, high...
04. June 2018
The selection of the excipient(s) for a tablet formulation should take into account the API properties, process, target formulation and potential impact on the formulation. Some of those API properties can be Dose Particle Size Flow Properties Bulk Density Moisture Content Hygroscopicity Excipient Compatibility Compactability The following tables show some possible impacts on the tablet formulation and considerations to take into account. Pharmaexcipients.com thanks Fred Monsuur, Grace for the...
24. September 2017
In the interest of saving time and reducing costs, drug developers are automating the screening process when selecting excipients for solubility. Evaluating the pharmacological, pharmacokinetical and toxicological properties of a drug candidate during early stages of drug development requires developments of liquid formulations. In order to solubilize compounds that are poorly soluble, solubilizing excipients need to be added. This needs to be done without compromising the concentration of the...
07. April 2017
Abstract A compound, which is a selective peroxisome proliferator activated receptor (PPAR) agonist, was investigated. The aim of the presented studies was to evaluate the potential of the further development of the compound. Fundamental physicochemical properties and stability of the compound were characterized in solution by liquid chromatography and NMR and in solid-state by various techniques. The drug itself is a poorly soluble lipophilic acid with tendency to form aggregates in solution....
11. January 2017
Abstract With the implementation of quality by design (QbD), critical attributes of raw material (drug substance and excipients) are of significantly importance in pharmaceutical manufacturing process. It is desirable for the quality control of critical material attributes (CMAs) of excipients to ensure the quality of end product. This paper explored the feasibility of an at-line method for the quantitative analysis of hydroxypropoxy group in hydroxypropyl methylcellulose (HPMC) with near...
18. December 2015
Pharmaceutical formulations have to fulfil various requirements with respect to their intended use, either in the development phase or as a commercial product. New drug candidates with their specific properties confront the formulation scientist with industrial challenges for which a strategy is needed to cope with limited resources, stretched timelines as well as regulatory requirements. More
17. February 2015
Ticehurst, M. D. and Marziano, I. (2015), Integration of active pharmaceutical ingredient solid form selection and particle engineering into drug product design. Journal of Pharmacy and Pharmacology. doi: 10.1111/jphp.12375 http://onlinelibrary.wiley.com/doi/10.1111/jphp.12375/abstract