- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
01. October 2017
In general, it is an important criterion that excipients remain inert throughout the shelf life of the formulated pharmaceutical product. However, depending on the functionality in chemical structure of active drug and excipients, they may undergo interaction. The well-known Maillard reaction occurs between a primary amine with lactose at high temperature to produce brown pigments. The reactivity of Maillard reaction may vary depending on the concentration as well as other conditions....
01. October 2017
Technologies for long-term delivery of aerosol medications in asthma and chronic obstructive pulmonary disease have improved over the past 2 decades with advancements in our understanding of the physical chemistry of aerosol formulations, device engineering, aerosol physics, and pulmonary biology. However, substantial challenges remain when a patient is required to use multiple inhaler types, multiple medications, and/or combinations of medications. Combining multiple drugs into a single...
01. October 2017
Orally disintegrating tablets (ODTs) are becoming increasingly important in the global pharmaceutical market for both prescription and over-the-counter medications because they can significantly improve patient compliance. They can be swallowed without the need for water, are generally smaller, and have good mouthfeel. Such properties make ODTs particularly convenient for children and the elderly, especially when a flavor is also incorporated into the formulation. In addition, they are the...
01. October 2017
Vitamin E TPGS (TPGS) has both surfactant and P-glycoprotein (P-gp) inhibitory effects. While surfactants were previously found to cause solubility-permeability tradeoff, TPGS P-gp inhibitory effects may change this unfavorable interplay. The purpose of this research was to investigate the solubility-permeability interplay when using TPGS vs. amorphous solid dispersions (ASD) as oral drug delivery systems for the anticancer, P-gp substrate, lipophilic drug etoposide. The concentration-dependent...
01. October 2017
Recently, we have introduced fibrous dosage forms prepared by the predictable deposition, or 3D-micro-patterning, of a drug-laden fibrous melt on a surface. Such dosage forms enable precisely controlled microstructures and drug release rates, and can be manufactured by an efficient, continuous melt process. However, the applicability of melt-processing to manufacture pharmaceutical dosage forms is limited because the temperatures at which suitable excipients plasticize by melting are greater...
30. September 2017
Taste is the maximum valuable factor within the case of orally drugs administering. Flavor covering is a prerequisite for bitter tablets to better the patient compliance, particularly in the paediatrics and geriatric population. The hassle of the sour taste of drug in pediatric formulations is a completely huge task to the formulators on this gift time. Overlaying is an effective device for the development of the sour taste of medication of patient compliance which sooner or later comes to a...
30. September 2017
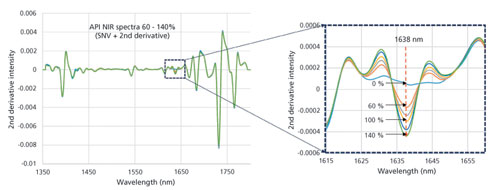
Within the pharmaceutical industry, inline near infrared (NIR) spectroscopy is one of the most commonly applied process analytical technologies to monitor content uniformity of powder blends (1). Inline NIR provides direct insight into blending operations with respect to the degree of uniformity of the ingredient of interest either for process development or control during commercial manufacturing. A quantitative calibration for a specific ingredient may be applied to predict the content of...
29. September 2017
The present article provides a novel technique for the co-amorphous formation of acyclovir and oxalic acid as an excipient. Designated as ACV-oxalic acid, methods for the preparation thereof and its use in pharmaceutical applications are described.
29. September 2017
Ibuprofen is a non-steroidal anti-inflam-matory drug frequently administered to children of var-ious ages for relief of fever and pain and is approved as over-the-counter medication in many countries world-wide. Although there is extensive data on its efficacy and safety in children and adults, there are divergent dosing recommendations for analgesia and treatment of fever in infants, especially in the age group between 3 and 6 months of age.
29. September 2017
An updated version of the Therapeutic Goods (Permissible Ingredients) Determination was registered on the Federal Register of Legislation (FRL) in September 2017. The updated determination is titled the Therapeutic Goods (Permissible Ingredients) Determination No. 4 of 2017.