- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
18. April 2018
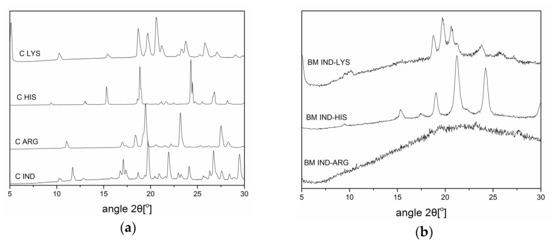
Ball-milling is usually used to prepare co-amorphous drug–amino acid (AA) mixtures. In this study, co-amorphous drug–AA mixtures were produced using spray-drying, a scalable industrially preferred preparation method. The influence of the solvent type and solvent composition was investigated. Mixtures of indomethacin (IND) and each of the three AAs arginine, histidine, and lysine were ball-milled and spray-dried at a 1:1 molar ratio, respectively. Spray-drying was performed at different...
10. February 2018
Pulmonary delivery of protein therapeutics has considerable clinical potential for treating both local and systemic diseases. However, poor protein conformational stability, immunogenicity and protein degradation by proteolytic enzymes in the lung are major challenges to overcome for the development of effective therapeutics.
26. January 2018
The understanding of amorphous solid dispersions has grown significantly in the past decade. This is evident from the number of approved commercial amorphous solid dispersion products. While amorphous formulation is considered an enabling technology, it has become the norm for formulating poorly soluble compounds.
25. October 2017
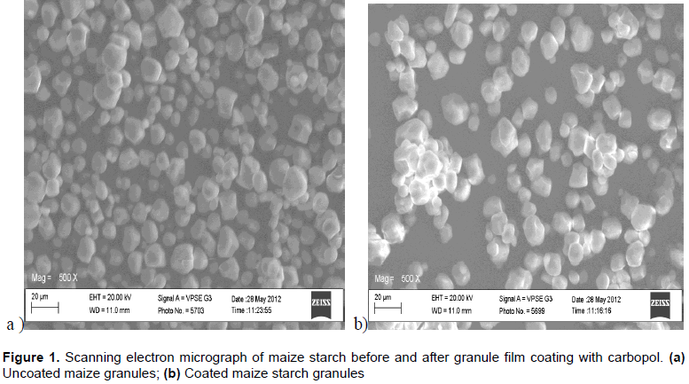
The aim of this study was to enhance the classical adjuvant functionality of maize starch (Mst) by co-processing with small quantities of carbopol 974P (CaPol). A pre-processed maize starch (MpS) was coated with different concentrations of CaPol corresponding to 0.25, 0.5, 0.75 and 1.0 %w/w and spray-dried at controlled conditions to produce the CaPol-coated motifs (MpS-CaPol).
29. May 2017
Self-emulsifying drug delivery systems (SEDDS) are lipid formulations that improve solubility and oral bioavailability of the incorporated drug with poor biopharmaceutical properties. As liquids they are traditionally filled into soft or hard capsules.
18. May 2017
Spray drying solutions for small molecule and biological therapeutics.
28. April 2017
The rising demand for pharmaceutical particles with tailored physico-chemical properties has opened new markets for the spray drying technology especially for solubility enhancement, improvement of inhalation medicines and stabilization of biopharmaceuticals. Despite this, the literature on spray drying is scattered and often does not address the fundamental principles underpinning the robust development of pharmaceutical products. It is therefore necessary to present a clearer picture of the fi
03. January 2017
ABSTRACT: Efavirenz is a crystalline lipophilic solid with a low aqueous solubility and intrinsic dissolution rate. It is classified in class II of the Biopharmaceutics Classification System, which means it is poorly water-soluble and highly permeable. Spray drying is a widely used manufacturing process wich uses the aerosol phase to dry particles. By modifying the spray drying operation parameters, it is possible to control the properties of spray dried particles towards enhancement of drug...
20. November 2016
Abstract Associating protein with nanoparticles is an interesting strategy to improve their bioavailability and biological activity. Solid lipid nanoparticles (SLN) have been sought as carriers for therapeutic proteins transport to the lung epithelium. Nevertheless, because of their low inertia, nanoparticles intended for pulmonary application usually escape from lung deposition. To overcome this problem, the production of spray-dried powders containing nanoparticles has been recently reported....
28. September 2016
Abstract Lipid nanoparticles and their multiple designs have been considered appealing nanocarrier systems. Bringing the benefits of these nanosystems together with conventional coating technology clearly results in product differentiation.This work aimed at developing an innovative solid dosage form for oral administration based on tableting nanostructured lipid carriers (NLC), coated with conventional polymer agents. NLC dispersions co-encapsulating olanzapine and simvastatin (Combo-NLC) were...