- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
03. September 2018
Nowadays, the freeze-drying of liposome dispersions is still necessary to provide a solid dosage form intended for different routes of administration (i.e., parenteral, oral, nasal and/or pulmonary). However, after decades of studies the optimization of process conditions remains still challenging since the freezing and the dehydration destabilize the vesicle organization with the concomitant drug leakage. Starting from the thermal properties of phospholipids, this work reviews the main...
30. August 2018
Hard gelatin capsule (HGC) shells are widely used to encapsulate drugs for oral delivery, but are vulnerable to gelatin cross-linking, which can lead to slower and more variable in vitro dissolution rates. Adding proteolytic enzymes to the dissolution medium can attenuate these problems, but this complicates dissolution testing and is only permitted by some regulatory authorities. Here, we expand the scope of our previous work to demonstrate that canisters containing activated carbon (AC) or...
30. August 2018
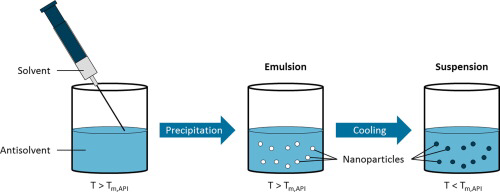
Antisolvent precipitation of poorly water-soluble drugs is a promising formulation technique to synthesize amorphous nanoparticles. The dissolution behavior of these nanoparticles is improved because of the high specific surface area and the amorphous state, leading to an enhanced bioavailability of the drug molecules. Nevertheless, stabilization of precipitated drug nanoparticles against agglomeration and recrystallization, which constitutes a key issue for further processing steps, has turned...
29. August 2018
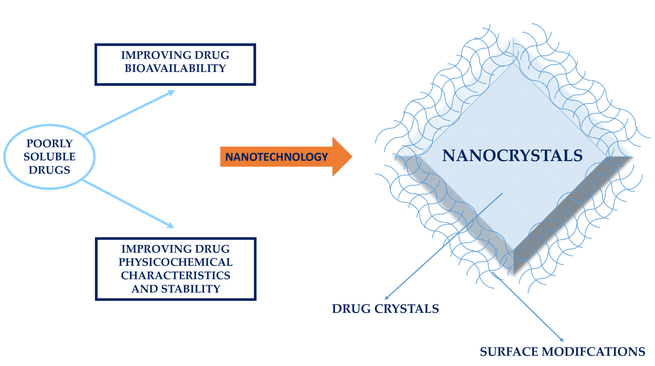
Many approaches have been developed over time to overcome the bioavailability limitations of poorly soluble drugs. With the advances in nanotechnology in recent decades, science and industry have been approaching this issue through the formulation of drugs as nanocrystals, which consist of “pure drugs and a minimum of surface active agents required for stabilization”. They are defined as “carrier-free submicron colloidal drug delivery systems with a mean particle size in the nanometer...
04. August 2018
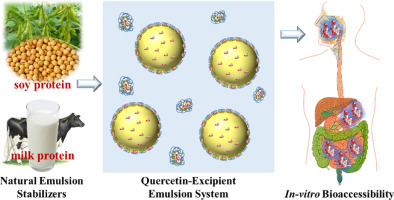
Emulsion-based excipient foods were developed to improve the bioaccessibility of an important hydrophobic nutraceutical: quercetin. Protein-stabilized oil-in-water excipient emulsions were prepared using sodium caseinate, whey protein isolate, or soy protein isolate as an emulsifier. These emulsions were then mixed with powdered quercetin and heated to simulate a cooking process. The excipient emulsions had relatively small droplet sizes (d < 270 nm) and remained stable against...
27. July 2018
The present study elucidated the advantages of using hydroxypropyl methylcellulose E5 (HPMC) as auxiliary excipient in maintaining storage stability and solubilization ability for curcumin amorphous solid dispersion (Cur ASDs) formulated by Eudragit E100 (E100). Polarized light microscopy and in vitro dissolution experiment was applied for confirming the ability of HPMC on inhibiting crystallization thereby maintaining storage stability in Cur ASDs. Meanwhile, as a non-ionic surfactant, HPMC...
08. July 2018
The purpose of this study was to develop self-microemulsifying (SME-) tablets to improve resveratrol solubility whilst delivering resveratrol in a preferred tablet dosage form. Resveratrol was dissolved in liquid self-microemulsifying drug delivery system (SMEDDS) (10 % w/w) and solidified through adsorption on several different solid carriers. Two ranges of synthetic amorphous silica (Sylysia® 290, 350, 470, 580; Syloid® 244FP, AL-1FP) as well as granulated magnesium aluminometasilicate...
04. July 2018
Concentrated solutions of doxycycline are often added to drinking water of animals for oral antibiotic therapy. However, stability concerns of doxycycline in solution involve an accurate selection of the solvent system to ensure that the active substance will remain within the acceptance range during the product shelf-life and to avoid sub-therapeutic dosage. Different solvent systems have been evaluated in order to determine their influence on the stability of concentrated doxycycline...
11. May 2018
The purpose of this study was to investigate if AZD5329, a dual neurokinin NK1/2 receptor antagonist, is a suitable candidate for further development as an oral immediate release (IR) solid dosage form as a final product. The neutral form of AZD5329 has only been isolated as amorphous material. In order to search for a solid material with improved physical and chemical stability and more suitable solid-state properties, a salt screen was performed. Crystalline material of a maleic acid salt and...
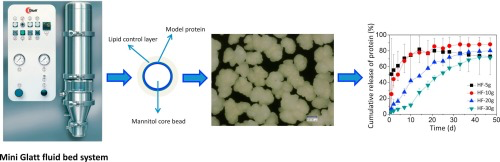
25. April 2018
Parenteral sustained release systems for proteins which provide therapeutic levels over a longer period avoiding frequent administration, which preserve protein stability during manufacturing, storage and application and which are biodegradable and highly biocompatible in the body are intensively sought after. The aim of this study was to generate and study mannitol core microparticles loaded with a monoclonal antibody IgG1 and coated with lipid either hard fat or glyceryl stearate at different...