- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
28. September 2017
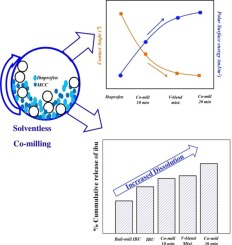
The wetting and dissolution of a BCS class II drug (Ibuprofen) is enhanced by solventless solid dispersion technique using co-milling. The co-milling is performed in a planetary ball mill using 1:1 wt. ratio of Ibuprofen (drug) and Microcrystalline cellulose, MCC (excipient).The improvement in wettability and dissolution after co-milling are compared with the raw ibuprofen, ball-milled ibuprofen without any excipient and v-blend mixture of ibuprofen with an excipient. The changes in crystal...
15. June 2016
Abstract In this study, terahertz time-domain spectroscopic (THz-TDS) technique has been used to ascertain the change in the optical properties, as a function of changing porosity and mass fraction of active pharmaceutical ingredient (API), of training sets of pharmaceutical tablets. Four training sets of pharmaceutical tablets were compressed with microcrystalline cellulose (MCC) excipient and indomethacin API by varying either the porosity, height, and API mass fraction or all three tablet...
04. June 2016
A full factorial design of experiments was used to study the effect of blend shear strain on the compaction process, relative density and strength of pharmaceutical tablets. The powder blends were subjected to different shear strain levels (integral of shear rate with respect to time) using an ad hoc Couette shear cell. Tablets were compressed at different compaction forces using an instrumented compactor simulator, and compaction curves showing the force-displacement profiles during compaction...
16. February 2016
Disintegrant is one of the most important components in a typical tablet dosage form. It is responsible for ensuring the break-up of the tablet matrix upon ingestion. Disintegrants act by different mechanisms, and a number of factors may affect their performance. It is important for formulators to understand how disintegrants function so as to be able to judiciously use disintegrants to develop optimized formulations. If the formulator is required to implement the quality by design paradigm...
11. August 2015
Capping is one of the major problems that occur during the tabletting process in the pharmaceutical industry. This study provided an effective method for evaluating the capping tendency during diametrical compression test using the finite element method (FEM). In experiments, tablets of microcrystalline cellulose (MCC) were compacted with a single tabletting machine, and the capping tendency was determined by visual inspection of the tablet after a diametrical compression test. More