- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
08. March 2018
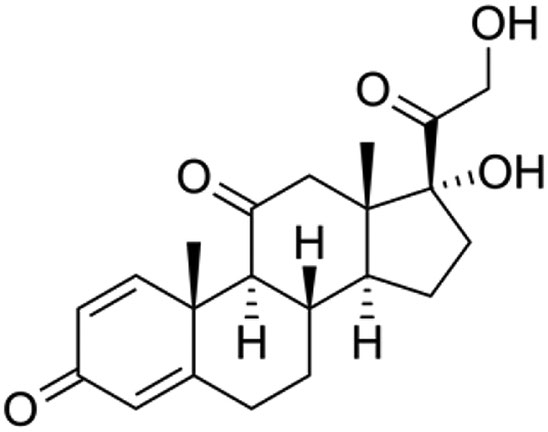
Prednisone is a corticosteroid used in several inflammatory diseases and cancers. In France, no available prednisone drinkable formulation exists. Instead, an oral syrup of prednisone with ethanol, sodium benzoate and simple syrup is produced. However, sodium benzoate can induce neonatal icterus and alcohol is not authorized for children below 3 years of age. The aim of this study was to determine the stability of 5 mg/mL prednisone oral suspension in a commercial compounding excipient: Syrspend
28. November 2017
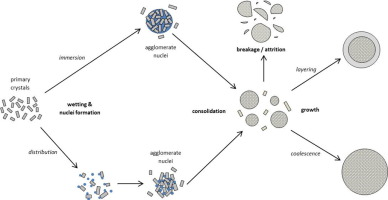
Particle design via spherical agglomeration is a size enlargement technique used in various bulk and fine chemical industries, with recent interest extending into pharmaceuticals, in which an immiscible bridging liquid is added to agglomerate crystals prior to deliquoring. Spherical agglomeration has the potential to dramatically simplify downstream processing, and improves the handling of difficult, needle-shaped crystals. This review consolidates the understanding of the controlling process...
19. July 2017
The objective of this study was to identify whether compounding oral suspensions with SyrSpend SF based on tablets or capsules is a suitable alternative for using raw pharmaceutical materials
17. January 2017
Abstract Excipients are the integral part of pharmaceutical product development to achieve the desired product profile (stability and efficacy). This review deals with understanding of the physicochemical properties of excipients used in parenteral formulation development for solution, suspension and lyophilized drug products. However, in spite of proper excipients selection, judicious use during formulation, manufacturing process based on their critical property that is also important to avoid...
17. January 2017
Excipients are the integral part of pharmaceutical products development to achieve desired product pro le (stability and efficacy). This review deals with understanding of the physicochemical properties of excipients used in parenteral formulation development for solution, suspension and lyophilized drug products. However, inspite of proper excipients selection, judicious use during formulation manufacturing process based on their critical properties is also important to avoid negative effects...
24. October 2016
Abstract The objective of this study was to evaluate the feasibility of lauryl sulfate (LS) salt/complex as a novel carrier in oral sustained-release suspensions. Mirabegron, which has a pH-dependent solubility, was selected as the model drug. Sodium lauryl sulfate (SLS) was bound to mirabegron in a stoichiometric manner to form a LS salt/complex. LS salt/complex formulation significantly reduced the solubility of mirabegron and helped mirabegron achieve sustained-release over a wide range of...
27. May 2016
Objective: Shea gum is found large quantities in the northern part of Ghana. Its use in the pharmaceutical industry has been limited by lack of research into the possible uses of the gum as a pharmaceutical excipient. This study seeks to investigate the use of shea gum as a suspending agent using paracetamol as a model drug. Method: The crude shea gum was collected, purified and used as a suspending agent to formulate paracetamol suspensions using gum concentrations of 1 %w/v, 2 %w/v, 3 % w/v...
11. May 2016
Excipients are the integral part of pharmaceutical product development to achieve the desired product profile (stability and efficacy). This review deals with understanding of the physicochemical properties of excipients used in parenteral formulation development for solution, suspension and lyophilized drug products. However, in spite of proper excipients selection, judicious use during formulation, manufacturing process based on their critical property that is also important to avoid negative...
13. April 2016
Objectives In this review paper, we explore the interaction between the functioning mechanism of different nebulizers and the physicochemical properties of the formulations!!! for several types of devices, namely jet, ultrasonic and vibrating-mesh nebulizers; colliding and extruded jets; electrohydrodynamic mechanism; surface acoustic wave microfluidic atomization; and capillary aerosol generation.
05. February 2016
Excipients are the integral part of pharmaceutical product development to achieve the desired product profile (stability and efficacy). This review deals with understanding of the physicochemical properties of excipients used in parenteral formulation development for solution, suspension and lyophilized drug products. However, in spite of proper excipients selection, judicious use during formulation, manufacturing process based on their critical property that is also important to avoid negative...