- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
18. July 2018
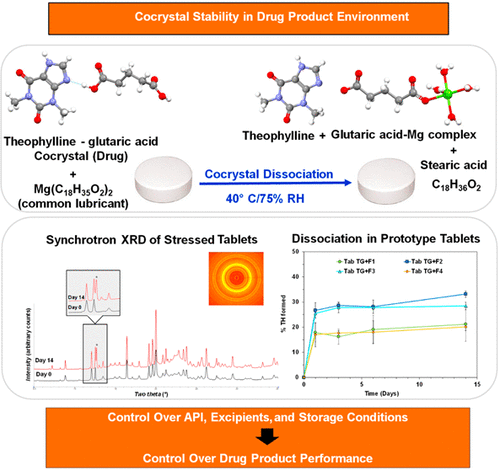
Tablets containing a theophylline–glutaric acid (TG) cocrystal dissociated rapidly forming crystalline theophylline (20–30%), following storage at 40 °C/75% RH for 2 weeks. Control tablets of TG cocrystal containing no excipients were stable under the same conditions. The dissociation reaction was water-mediated, and the theophylline concentration (the dissociation product), monitored by synchrotron X-ray diffractometry, was strongly influenced by the formulation composition. Investigation...
04. June 2018
The selection of the excipient(s) for a tablet formulation should take into account the API properties, process, target formulation and potential impact on the formulation. Some of those API properties can be Dose Particle Size Flow Properties Bulk Density Moisture Content Hygroscopicity Excipient Compatibility Compactability The following tables show some possible impacts on the tablet formulation and considerations to take into account. Pharmaexcipients.com thanks Fred Monsuur, Grace for the...