- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
01. September 2018
Flow properties of microcrystalline cellulose (MCC) excipients, Avicel PH 101 and Avicel PH 102 have been compared by using Brookfield PFT. Afterwards, the effect of hydrophobic Silica R972 as glidant has been tested on both the excipients. Hand blending is done by mixing MCCs with hydrophobic silica R972 vigorously by SAC as an underlined basis for 10 minutes and the flow properties tests are performed. During the flow function test “as received” Avicel PH 102 shows better flow function...
01. May 2018
Downstream processing aspects of a stable form of amorphous itraconazole exhibiting enhanced dissolution properties were studied. Preparation of this ternary amorphous solid dispersion by either spray drying or hot melt extrusion led to significantly different powder processing properties. Particle size and morphology was analysed using scanning electron microscopy. Flow, compression, blending and dissolution were studied using rheometry, compaction simulation and a dissolution kit. The spray...
04. September 2016
Abstract: This paper focuses on the characterization of the tabletting process and analysis one of the most common pharmaceutical excipients MCC Avicel PH102 by Heckel, Kawakita, Cooper-Eaton and Adams compaction equations. Experimental material was determined by measuring its parameters as particle size distribution, angle of wall friction and flow properties and for more detailed characteristics of the material particles, microscopy images of the powder before and after compressing were...
29. March 2016
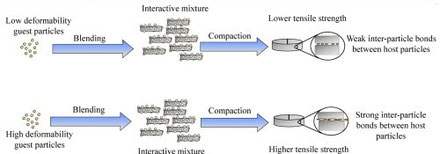
In this study, we investigated the influence of deformability of specifically-engineered guest particles on the tensile strength of tablets of interactive mixtures. The binder polyvinylpyrrolidone (PVP) of different molecular weights were spray dried with l-leucine to create guest particle formulations. The guest particle formulations were characterized by their particle size, surface l-leucine concentration and glass transition temperature (Tg). These spray-dried particles were then blended...
16. February 2016
In the pharmaceutical field, solid-state transitions that may occur during manufacturing of pharmaceuticals are of great importance. The phase transition of a model API, caffeine Form I (CFI), was studied during direct compression process by analysing the impacts of the operating conditions (process and formulation). This work is focused on two formulation parameters: nature of the diluent and impact of the caffeine dilution, and one process parameter: the compression pressure that may impact...
11. August 2015
Capping is one of the major problems that occur during the tabletting process in the pharmaceutical industry. This study provided an effective method for evaluating the capping tendency during diametrical compression test using the finite element method (FEM). In experiments, tablets of microcrystalline cellulose (MCC) were compacted with a single tabletting machine, and the capping tendency was determined by visual inspection of the tablet after a diametrical compression test. More