- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
18. August 2018
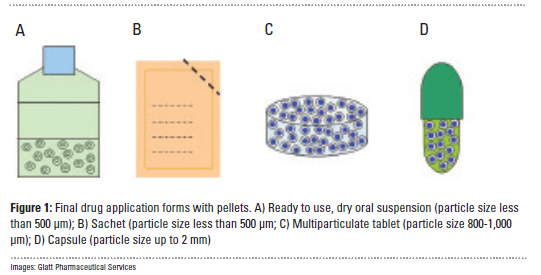
In contrast to classic single-unit dosage forms such as tablets, the dosage of the drug substance in multi-particulate systems is divided on a plurality of subunits – typically consisting of thousands of spherical pellet particles with a diameter of between 100 and 2,000 μm. This means that non-disintegrating, monolithic single-unit forms retain their structure in the digestive tract, whereas the multi-particular preparations consist of numerous sub-units which disperse after administration....
02. July 2018
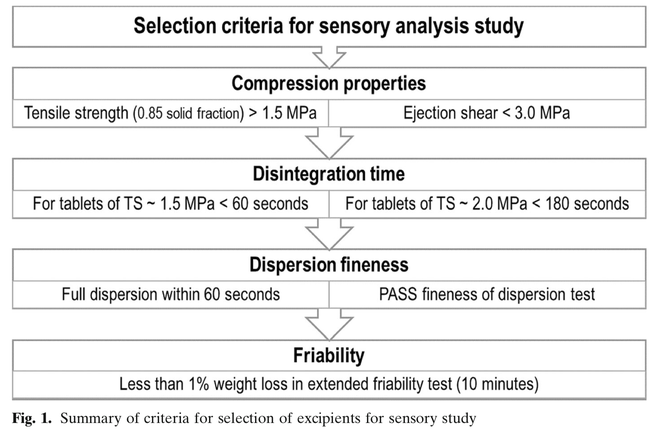
Palatability and patient acceptability are critical attributes of dispersible tablet formulation. Co-processed excipients could provide improved organoleptic profile due to rational choice of excipients and manufacturing techniques. The aim of this study was to identify the most suitable co-processed excipient to use within directly compressible dispersible tablet formulations. Nine excipients, selected based on successful manufacturability, were investigated in a randomised, preference and...
11. May 2018
Improve Compliance by Optimizing the Taste of Active Compounds Dietary supplements for athletes and balanced additives for all age groups must, above all, possess one property: they have to taste good. Consumer acceptance is essential for long-term use and thus for the desired, positive effect on performance and health. However, many active substances do not taste nice. Their tastes range from sulphurous to fishy to astringent and bitter. Alpha-lipoic acid, caffeine or resveratrol are just a...
24. December 2017
Drug therapy for children is one of the cornerstone developments that have sharply reduced childhood mortality. Despite this, many challenges remain in ensuring that children receive safe and effective drug therapy. There are unique issues in treating children with oral medication relating to development, existing formulations and medication acceptability. Medication acceptability in children is complex relating to a wide range of factors, including drug palatability.
26. September 2017
The recommended first-line treatment for young children infected with HIV includes the liquid formulation of the co-formulated protease inhibitors lopinavir/ritonavir (Kaletra® [Abbott Laboratories, Chicago, Illinois]). Clinical reports indicate that some children readily accept the taste of Kaletra, whereas others strongly reject it, which can deter therapeutic adherence and outcomes.
14. September 2017
Prof Martin Whitaker (The University of Sheffield) will present Infacort®: a PUMA success story at the 9th conference of the European Paediatric Formulation Initiative (EuPFI) in Warsaw (Poland) on Thursday 21st September 2017 at 12:25.
30. June 2017
When developing an oral liquid dosage formulation, consideration is first given to the characteristics of the active drug. The major challenges in developing oral liquid dosage forms are (i) the stability of a drug in solution, (ii) the solubility of a drug at the required level, and (iii) an acceptable taste.
20. April 2017
Abstract Optimized orally disintegrating tablets (ODTs) containing furosemide (FUR) were prepared by direct compression method. Two factors, three levels (32) full factorial design was used to optimize the effect of taste masking agent (Eudragit E100; X1) and superdisintegarant; croscarmellose sodium (CCS; X2) on tablet properties. A composite was prepared by mixing ethanolic solution of FUR and Eudragit E100 with mannitol prior to mixing with other tablet ingredients. The prepared ODTs were...
04. April 2017
Abstract The objective of this study was to prepare and evaluate some physiochemical and biopharmaceutical properties of bitter taste masking microparticles containing azithromycin loaded in dispersible tablets. In the first stage of the study, the bitter taste masking microparticles were prepared by solvent evaporation and spray drying method. When compared to the bitter threshold (32.43 µg/ml) of azithromycin (AZI), the microparticles using AZI:Eudragit L100 = 1:4 and having a size...
20. March 2017
Abstract In the early development stage of orally disintegrating films (ODFs), suitable evaluation methods must be used. The aim of this study was to demonstrate the feasibility of using an electronic taste sensor and a flow-through cell for ODFs. A pullulan film loaded with donepezil hydrochloride (DH) was designed, as a model ODF. The film was prepared using the solution/solvent casting method. First, the dissolution profiles were compared by using a paddle method (PD) and a flow-through cell...