- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. July 2018
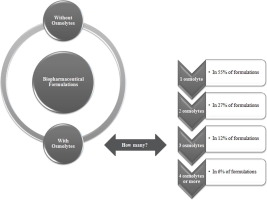
Osmolytes are small organic molecules accumulated by cells in response to environmental stresses. They are represented by amino acids, sugars, polyols, tertiary sulphonium and quaternary ammonium compounds. These molecules present a protective behaviour and favour the equilibrium of macromolecules towards the native form, preventing denaturation and promoting the folding of unfolded proteins. Protein formulations due to their biological character require greater care during the manufacturing...
13. April 2018
Current global vaccination programs are challenged by costs associated with vaccine cold chain storage and administration. A solid, thermally stable oral dosage form for vaccines would alleviate these costs but is difficult to produce due to general vaccine instability and the complication of bypassing the gastric barrier. We developed a novel consecutive spray drying method that is suitable for use with biologics and employs Eudragit L100 polymer as the enteric coating. More specifically, in...
11. November 2017
The following table lists all components, other than antigens, shown in the manufacturers’ package insert (PI) for each vaccine. Each of these PIs, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance.
14. October 2017
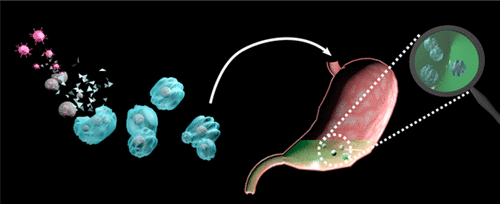
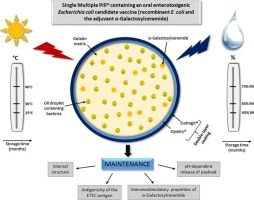
Oral vaccines present an attractive alternative to injectable vaccines for enteric diseases due to ease of delivery and the induction of intestinal immunity at the site of infection. However, susceptibility to gastrointestinal proteolysis, limited transepithelial uptake and a lack of clinically acceptable adjuvants present significant challenges. A further challenge to mass vaccination in developing countries is the very expensive requirement to maintain the cold chain. We recently described...
15. December 2016
Abstract Most current vaccine preparations are in injectable forms, which are inconvenient to patients and ineffective in mucosal immunization. Therefore, most research in this field has been directed at developing ideal oral vaccines enabling the induction of both systemic and mucosal immune responses. In the present study, we examined the utility of a pH-responsive polymeric carrier, poly (methacrylic acid-g-ethylene glycol) [P (MAA-g-EG)] hydrogel, as a potential oral vaccine carrier that...
01. May 2016
We have produced a thermally stable recombinant human type 5 adenoviral vector (AdHu5) through spray drying with three excipient formulations (l-leucine, lactose/trehalose and mannitol/dextran). Spray drying leads to immobilization of the viral vector which is believed to prevent viral protein unfolding, aggregation and inactivation. The spray dried powders were characterized by scanning electron microscopy, differential scanning calorimetry, Karl Fischer titrations, and X-ray diffraction to...
21. February 2016
Excipients Included in U.S. Vaccines, by Vaccine This table includes not only vaccine ingredients (e.g., adjuvants and preservatives), but also substances used during the manufacturing process, including vaccine-production media, that are removed from the final product and present only in trace quantities. In addition to the substances listed, most vaccines contain Sodium Chloride (table salt).Last Updated February 2015 Pink Book Wikipedia Link
17. February 2016
A number of biobarriers limit efficient oral drug absorption; both polymer-based and lipid-based nanocarriers have demonstrated properties and delivery mechanisms to overcome these biobarriers in preclinical settings. Moreover, in order to address the multifaceted oral drug delivery challenges, polymer-lipid hybrid systems are now being designed to merge the beneficial features of both polymeric and lipid-based nanocarriers. More
17. February 2016
Dry powder formulations for nasal vaccine delivery offer versatile advantages compared to liquid formulations, such as increased storage stability and simplified administration. The objective of the present study was the development of a dry powder nasal vaccine formulation making use of antigen-loaded chitosan microparticles. Special emphasis was put on the development and characterization of a formulation which can realistically be used in humans by means of a nasal dry powder sprayer....
25. January 2016
Recently, we reported that intranasal vaccination of humans with whole inactivated influenza vaccine in the absence of mucosal adjuvant induced neutralizing antibody responses in the serum and nasal mucus. The mucoadhesive excipient carboxy-vinyl polymer (CVP) increases the viscosity and therefore mucoadhesiveness of intranasal medicaments and is an authorized excipient in Japan. In the present study, we analyzed the effect of adding CVP on intranasal whole inactivated influenza vaccine antigen...