- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
10. January 2018
Faecalibacterium prausnitzii was previously recognized for its intestinal anti-inflammatory activities and it has been shown less abundant in patients with chronic intestinal diseases. However, the main problems encountered in the use of this interesting anaerobic microorganism are firstly its high sensitivity to the oxygen and secondly, its ability to reach the large intestine alive as targeted site.
08. October 2017
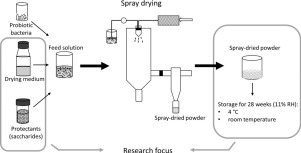
Increasing knowledge about the human microbiome has led to a growing awareness of the potential of applying probiotics to improve our health. The pharmaceutical industry shows an emerging interest in pharmaceutical formulations containing these beneficial microbes, the so-called pharmabiotics. An important manufacturing step is the drying of the probiotics, as this can increase the stability and shelf life of the finished pharmabiotic product. Unfortunately, drying also puts stress on microbial...
17. October 2016
Abstract The present study focused on the preparation, physico-chemical, and biological characterization of several composite films containing graphene derivatives (graphene oxide or reduced graphene oxide) and curcumin as antioxidant in methylcellulose matrix. The morphostructural properties of the obtained composites were investigated by scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier transformed infrared spectroscopy (FTIR), and X-ray powder diffraction (XRD). The...
28. April 2016
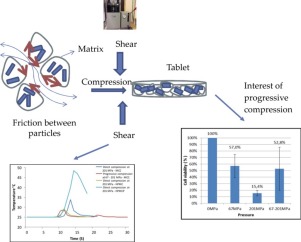
Abstract: The aim of this study was to formulate probiotics-loaded pellets in a tablet form to improve storage stability, acid tolerability, and in vivo intestinal protective effect. Bacteria-loaded pellets primarily prepared with hydroxypropyl methylcellulose acetate succinate were compressed into tablets with highly compressible excipients and optimized for flow properties, hardness, and disintegration time. The optimized probiotic tablet consisted of enteric-coated pellets (335 mg),...
18. October 2015
Carboxymethyl starch microspheres (CMS-MS) were produced from carboxymethyl starch powder (CMS-P) with a degree of substitution (DS) from 0.1 to 1.5 in order to investigate the influence of DS on physicochemical, drug release and mucoadhesion properties as well as interactions with gastrointestinal tract (GIT) epithelial barrier models. More